Epstein-Barr virus induces host shutoff extensively via BGLF5-independent mechanisms
- PMID: 39298313
- PMCID: PMC11549945
- DOI: 10.1016/j.celrep.2024.114743
Epstein-Barr virus induces host shutoff extensively via BGLF5-independent mechanisms
Abstract
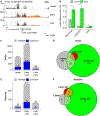
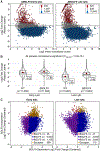
Epstein-Barr virus (EBV) is a ubiquitous oncogenic virus associated with multiple cancers and autoimmune diseases. Unlike most herpesviruses, EBV reactivation from latency occurs asymptomatically, allowing it to spread efficiently to other hosts. However, available models are limited by the inefficient and asynchronous reactivation from latency into lytic replication. To address this problem, we develop a dual-fluorescent lytic reporter (DFLR) EBV that specifically labels cells in the early and late stages of replication. Using lymphoblastoid cell lines transformed by DFLR EBV as a model for EBV reactivation in B cells, we observe extensive reprogramming of the host cell transcriptome during lytic cycle progression. This includes widespread shutoff of host gene expression and disruption of mRNA processing. Unexpectedly, host shutoff remains extensive even in cells infected with DFLR EBV deleted for the BGLF5 nuclease. These findings implicate BGLF5-independent mechanisms as the primary drivers of host transcriptome remodeling during EBV lytic replication.
Keywords: CP: Immunology; CP: Microbiology; EBV; herpesvirus; host shutoff; lytic reactivation; splicing; transcriptomics; tumor virus.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





References
-
- Johannsen E, and Kaye K (2020). Epstein-Barr Virus (Infectious Mononucleosis, Epstein-Barr Virus-Associated Malignant Diseases, and Other Diseases). In Mandell, Douglas, and Bennett’s Principles and Practice of Infectious Diseases, Bennett J, Dolin R, and Blaser M, eds. (Elsevier; ), pp. 1872–1890.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

