Daily oscillations of neuronal membrane capacitance
- PMID: 39298314
- PMCID: PMC11744780
- DOI: 10.1016/j.celrep.2024.114744
Daily oscillations of neuronal membrane capacitance
Abstract
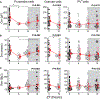
Capacitance of biological membranes is determined by the properties of the lipid portion of the membrane as well as the morphological features of a cell. In neurons, membrane capacitance is a determining factor of synaptic integration, action potential propagation speed, and firing frequency due to its direct effect on the membrane time constant. Besides slow changes associated with increased morphological complexity during postnatal maturation, neuronal membrane capacitance is considered a stable, non-regulated, and constant magnitude. Here we report that, in two excitatory neuronal cell types, pyramidal cells of the mouse primary visual cortex and granule cells of the hippocampus, the membrane capacitance significantly changes between the start and the end of a daily light-dark cycle. The changes are large, nearly 2-fold in magnitude in pyramidal cells, but are not observed in cortical parvalbumin-expressing inhibitory interneurons. Consistent with daily capacitance fluctuations, the time window for synaptic integration also changes in pyramidal cells.
Keywords: CP: Neuroscience; Circadian; capacitance; cortex; electrophysiology; granule cells; hippocampus; oscillators; pyramidal cells.
Copyright © 2024 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
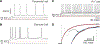
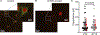
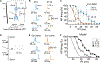
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

