Steroid-refractory immune checkpoint inhibitor (ICI) hepatitis and ICI rechallenge: A systematic review and meta-analysis
- PMID: 39298568
- PMCID: PMC11412713
- DOI: 10.1097/HC9.0000000000000525
Steroid-refractory immune checkpoint inhibitor (ICI) hepatitis and ICI rechallenge: A systematic review and meta-analysis
Abstract
Background: In recent years, the use of immune checkpoint inhibitors (ICIs) has become a cornerstone in cancer treatment. However, this has also resulted in the emergence of immune-related adverse events, notably ICI hepatitis, posing a significant clinical challenge. While steroids are the primary treatment, there are increasing cases of steroid-refractory ICI hepatitis. Our objective is to investigate the management of ICI hepatitis and its response to steroid treatment.
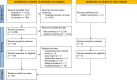
Methods: PubMed/MEDLINE, EMBASE, and CENTRAL databases were searched in July 2023 based on keywords including ICIs (anti-Programmed cell death protein 1/Programmed Death-Ligand 1, anti-CTLA-4, and anti-LAG3) and hepatitis.
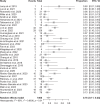
Results: A total of 4358 studies were screened, and 44 studies were included in this systematic review. One thousand eight hundred fifty-six patients with ICI hepatitis were included (grade 1-2: 31.7%, grade 3-4: 56.0%, and unknown: 12.3%) with 1184 patients who received corticosteroid treatment. The duration of treatment and dosage varied considerably across the studies. Mycophenolate mofetil was the predominant agent used in 68 out of 82 cases (82.9%), followed by infliximab and azathioprine. A summary estimate of the proportion of steroid-refractory hepatitis in a random effects model was 16% (95% CI: 11%-23%). An estimated 40% (95% CI: 30%-51%) of patients of all patients with ICI hepatitis were rechallenged with an ICI, and of those rechallenged, there was an estimated 22% (95% CI: 15%-30%) recurrence.
Conclusions: Corticosteroids are the primary treatment for ICI hepatitis, with mycophenolate mofetil used as a secondary option for steroids-refractory cases. Current practices mostly rely on expert consensus, highlighting the need for further research to validate and optimize these treatments, particularly for steroid-resistant cases.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Association for the Study of Liver Diseases.
Conflict of interest statement
The authors have no conflicts to report.
Figures

References
-
- Sullivan RJ, Weber JS. Immune-related toxicities of checkpoint inhibitors: Mechanisms and mitigation strategies. Nat Rev Drug Discov. 2022;21:495–508. - PubMed
-
- Haanen JBAG, Carbonnel F, Robert C, Kerr KM, Peters S, Larkin J, et al. . Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv119–iv142. - PubMed
-
- Dougan M, Wang Y, Rubio-Tapia A, Lim JK. AGA clinical practice update on diagnosis and management of immune checkpoint inhibitor colitis and hepatitis: Expert review. Gastroenterology. 2021;160:1384–1393. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

