Phase II Study of Samotolisib in Children and Young Adults With Tumors Harboring Phosphoinositide 3-Kinase/Mammalian Target of Rapamycin Pathway Alterations: Pediatric MATCH APEC1621D
- PMID: 39298693
- PMCID: PMC11581706
- DOI: 10.1200/PO.24.00258
Phase II Study of Samotolisib in Children and Young Adults With Tumors Harboring Phosphoinositide 3-Kinase/Mammalian Target of Rapamycin Pathway Alterations: Pediatric MATCH APEC1621D
Abstract
Purpose: Patients age 1-21 years with relapsed or refractory solid and CNS tumors were assigned to phase II studies of molecularly targeted therapies on the National Cancer Institute-Children's Oncology Group (NCI-COG) Pediatric Molecular Analysis for Therapy Choice (MATCH) trial. Patients whose tumors harbored predefined genetic alterations in the phosphoinositide 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) pathway and lacked mitogen-activated protein kinase pathway activating alterations were treated with the PI3K/mTOR inhibitor samotolisib.
Methods: Patients received samotolisib twice daily in 28-day cycles until disease progression or unacceptable toxicity. A rolling 6 limited dose escalation was performed as, to our knowledge, this was the first pediatric study of samotolisib. The primary end point was the objective response rate; secondary end points included progression-free survival (PFS) and the recommended phase II dose and toxicity of samotolisib in children.
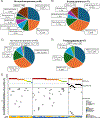
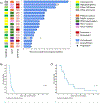
Results: A total of 3.4% (41/1,206) of centrally tested patients were matched to this arm. Seventeen patients were treated. Among treated patients, the most common diagnoses included osteosarcoma (n = 6) and high-grade glioma (n = 5) harboring alterations in phosphatase and tensin homolog (n = 6), PIK3CA (n = 5), and tuberous sclerosis complex 2 (n = 3). No objective responses or prolonged stable disease were observed. Three-month PFS was 12% (95% CI, 2 to 31). Two patients experienced dose-limiting toxicities (mucositis and pneumonitis). Dose level 2 (115 mg/m2/dose twice daily) was determined to be the recommended phase II dose of samotolisib in children.
Conclusion: This nationwide study was successful at identifying patients and evaluating the efficacy of molecularly targeted therapy for rare molecular subgroups of patients in a histology-agnostic fashion. Unfortunately, there was no activity of samotolisib against tumors with PI3K/mTOR pathway alterations. Prospective trials such as the NCI-COG Pediatric MATCH are necessary to evaluate the efficacy of molecularly targeted therapies given their increasing use in clinical practice.
Figures

References
-
- Harris MH, DuBois SG, Glade Bender JL, et al. : Multicenter Feasibility Study of Tumor Molecular Profiling to Inform Therapeutic Decisions in Advanced Pediatric Solid Tumors: The Individualized Cancer Therapy (iCat) Study. JAMA Oncol, 2016 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

