Intravenous Thrombolysis in Patients With Cervical Artery Dissection: A Secondary Analysis of the STOP-CAD Study
- PMID: 39298709
- PMCID: PMC11415265
- DOI: 10.1212/WNL.0000000000209843
Intravenous Thrombolysis in Patients With Cervical Artery Dissection: A Secondary Analysis of the STOP-CAD Study
Abstract
Objectives: Cervical artery dissection (CeAD) accounts for 25% of ischemic strokes in young adults. This study evaluated the benefits and harms of intravenous thrombolysis (IVT) in patients presenting with spontaneous CeAD and acute ischemic stroke symptoms.
Methods: This analysis used data from the retrospective STOP-CAD study and included patients with spontaneous CeAD who presented within 1 day of acute ischemic stroke symptoms. Patients were dichotomized into those who received IVT and those managed without IVT. We assessed the association between IVT and 90-day functional independence (modified Rankin Scale scores 0-2) and the incidence of symptomatic intracranial hemorrhage (ICH, defined as ICH causing new or worsening neurologic symptoms within 72 hours after CeAD diagnosis).
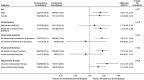
Results: This study included 1,653 patients from the original STOP-CAD cohort of 4,023. The median age was 49 years, and 35.1% were women; 512 (31.0%) received IVT. IVT was associated with 90-day functional independence (adjusted odds ratio [aOR] = 1.67, 95% CI 1.23-2.28, p = 0.001), but not with symptomatic ICH (aOR = 1.52, 95% CI 0.79-2.92, p = 0.215).
Discussion: In patients with spontaneous CeAD and suspected ischemic stroke, IVT improved functional outcomes, without increasing symptomatic ICH risk. These findings support current guideline recommendations to consider thrombolysis for otherwise eligible patients with CeAD.
Classification of evidence: This study provides Class III evidence that IVT significantly increases the probability of 90-day functional independence in patients with CeAD.
Conflict of interest statement
Disclosures provided by T. Nguyen in compliance with American Heart Association annual Journal Editor Disclosure Questionnaire are available at
Figures
References
-
- Yaghi S, Engelter S, Del Brutto VJ, et al.; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; and Council on Peripheral Vascular Disease. Treatment and outcomes of cervical artery dissection in adults: a scientific statement from the American Heart Association. Stroke. 2024;55(3):e91-e106. doi: 10.1161/STR.0000000000000457 - DOI - PubMed
-
- Engelter ST, Dallongeville J, Kloss M, et al.; Cervical Artery Dissection and Ischaemic Stroke Patients-Study Group. Thrombolysis in cervical artery dissection--data from the cervical artery dissection and ischaemic stroke patients (CADISP) database. Eur J Neurol. 2012;19(9):1199-1206. doi: 10.1111/j.1468-1331.2012.03704.x - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
