PEARL: A Phase Ib/II Biomarker Study of Adding Radiation Therapy to Pembrolizumab Before Neoadjuvant Chemotherapy in Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer
- PMID: 39298718
- PMCID: PMC12422141
- DOI: 10.1200/JCO.24.00003
PEARL: A Phase Ib/II Biomarker Study of Adding Radiation Therapy to Pembrolizumab Before Neoadjuvant Chemotherapy in Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer
Abstract
Purpose: To assess safety and immune biomarkers after preoperative radiation therapy (RT) and anti-PD1 therapy in breast cancer.
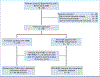
Materials and methods: A phase I/IIb trial of pembrolizumab with RT was conducted in patients with triple-negative breast cancer (TNBC) and hormone receptor-positive/human epidermal growth factor receptor 2-negative (HR+/HER2-) breast cancer. All received pembrolizumab followed by a second cycle + RT (anti-PD1/RT) of 24 Gy/three daily fractions delivered to the breast tumor and then neoadjuvant chemotherapy (NAC). Blood and tumor biopsies were obtained at baseline, after anti-PD1, and after anti-PD-RT. Coprimary end points were safety and change in tumor-infiltrating lymphocytes (TILs). Secondary end points were pathologic complete response (pCR), residual cancer burden (RCB) rates, and event-free survival (EFS).
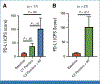
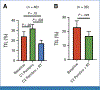
Results: Sixty-six patients with stage I-III breast cancer (54 TNBC, 12 HR+/HER2-) were enrolled. The median follow-up was 32 months. Safety end point was met. Incidence of grade ≥3 toxicities was 41%. The pCR rate was 59.2%, 33.3%, and 54.5% for the TNBC, HR+/HER2-, and entire cohort, respectively. A total of 77.8% of TNBC and 41.6% of HR+/HER2- had a near pCR (RCB 0-1). The 3-year EFS was 80%. In the entire cohort, PD-L1 expression increased after anti-PD1 (median Combined Positive Score [CPS], 7.49-23.20; 95% CI, -41.88 to -6.30; P = .044) and anti-PD1/RT (median CPS, 7.49-23.41; 95% CI, -41.88 to -6.30; P = .009), compared with baseline. In TNBC, adding RT to anti-PD1 significantly decreased TILs (28.9%-17.1%; 95% CI, 2.46 to 21.09; P = .014). Baseline TILs correlated with PD-L1 expression and TNF-a.
Conclusion: Preoperative RT with pembrolizumab is safe and results in high pCR rates and 3-year EFS, despite the lack of pembrolizumab during NAC. PD-L1 and TILs may be predictive biomarkers for preoperative anti-PD1/RT response. Reduction in TILs after adding RT to anti-PD1 highlights the importance of treatment sequencing.
Trial registration: ClinicalTrials.gov NCT03366844.
Conflict of interest statement
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO’s conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Alice Y. Ho
Stephen Shiao
Jonathan Chen
Dan G. Duda
Simon Knott
Gaorav P. Gupta
Philomena McAndrew
Scott Karlan
Reva Basho
Heather McArthur
No other potential conflicts of interest were reported.
Figures






References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

