Clinical Course of Neurologic Adverse Events Associated With Immune Checkpoint Inhibitors: Focus on Chronic Toxicities
- PMID: 39298719
- PMCID: PMC11413993
- DOI: 10.1212/NXI.0000000000200314
Clinical Course of Neurologic Adverse Events Associated With Immune Checkpoint Inhibitors: Focus on Chronic Toxicities
Abstract
Background and objectives: The clinical course and the risk of chronicity of neurologic immune-related adverse events (n-irAEs) associated with immune checkpoint inhibitors (ICIs) are not well documented. This study aimed to characterize the clinical course of n-irAEs and assess the prevalence of chronic events.
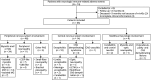
Methods: This nationwide, multicenter, retrospective study included patients with n-irAEs identified at 7 Italian hospitals. The clinical course of n-irAEs was categorized into fulminant (if resulted in death within 12 weeks), monophasic (if resolved within 12 weeks), and chronic (if persisted beyond 12 weeks). Chronic n-irAEs were further subdivided into active (if there was indirect evidence of ongoing inflammation [i.e., required ongoing immunosuppression, relapsed on steroid tapering, or exhibited neurologic progression]) and inactive (if patients had neurologic sequelae without ongoing inflammation). Comparisons between groups and time-to-death analyses were performed.
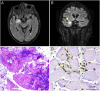
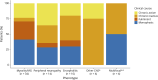
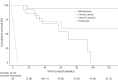
Results: Sixty-six patients were included (median age: 69 years [IQR 62-75]; 53 [80%] men). n-irAEs involved the peripheral nervous system in 48 patients (73%), the central nervous system in 14 (21%), and both in 4 (6%). Twelve patients (18%) had a fulminant course, with the risk being significantly higher in those with concurrent myocarditis (OR 5.4; 95% CI [1.02-28.31]). Among 54 patients with a nonfulminant course, 23 (43%) had a monophasic n-irAE and 31 (57%) had a chronic n-irAE, of which 16 of 31 (52%) were chronic active (due to ongoing immunosuppression [69%], relapses at corticosteroid tapering [19%], or neurologic disease progression [12%]) and 15 of 31 (48%) were chronic inactive. In patients with chronic inactive n-irAEs, neurologic sequelae included cerebellar ataxia (33%), neuromuscular weakness (27%), visual loss (13%), sensory disturbances (13%), focal neurologic signs (7%), and cognitive impairment (7%). Compared with patients with monophasic events, those with chronic n-irAEs had a higher rate of severe neurologic disability at the last evaluation (p < 0.01), shorter survival (p < 0.01), and higher overall mortality (p < 0.01), primarily due to cancer progression.
Discussion: More than half of the patients with n-irAEs who survived the acute phase developed a chronic condition. Patients with chronic n-irAEs were at higher risk of death, mainly due to cancer progression. Future studies are needed to further characterize chronic n-irAEs and identify optimal long-term management strategies.
Conflict of interest statement
A. Dinoto received research grants from Autoimmune Encephalitis Alliance and Encephalitis International, unrelated to the present study; SM received speaker honoraria from Horizon, UCB, Novartis, Biogen, Sanofi, Alexion, TSF, and Dynamics. Go to
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
