Risk of Recurrent Venous Thromboembolism in Patients with Cancer: An Individual Patient Data Meta-analysis and Development of a Prediction Model
- PMID: 39299270
- PMCID: PMC12115544
- DOI: 10.1055/a-2418-3960
Risk of Recurrent Venous Thromboembolism in Patients with Cancer: An Individual Patient Data Meta-analysis and Development of a Prediction Model
Abstract
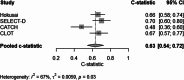
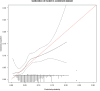
About 7% of patients with cancer-associated venous thromboembolism (CAT) develop a recurrence during anticoagulant treatment. Identification of high-risk patients may help guide treatment decisions.To identify clinical predictors and develop a prediction model for on-treatment recurrent CAT.For this individual patient data meta-analysis, we used data from four randomized controlled trials evaluating low-molecular-weight heparin or direct oral anticoagulants (DOACs) for CAT (Hokusai VTE Cancer, SELECT-D, CLOT, and CATCH). The primary outcome was adjudicated on-treatment recurrent CAT during a 6-month follow-up. A clinical prediction model was developed using multivariable logistic regression analysis with backward selection. This model was validated using internal-external cross-validation. Performance was assessed by the c-statistic and a calibration plot.After excluding patients using vitamin K antagonists, the combined dataset comprised 2,245 patients with cancer and acute CAT who were treated with edoxaban (23%), rivaroxaban (9%), dalteparin (47%), or tinzaparin (20%). Recurrent on-treatment CAT during the 6-month follow-up occurred in 150 (6.7%) patients. Predictors included in the final model were age (restricted cubic spline), breast cancer (odds ratio [OR]: 0.42; 95% confidence interval [CI]: 0.20-0.87), metastatic disease (OR: 1.44; 95% CI: 1.01-2.05), treatment with DOAC (OR: 0.66; 95% CI: 0.44-0.98), and deep vein thrombosis only as an index event (OR: 1.72; 95% CI: 1.31-2.27). The c-statistic of the model was 0.63 (95% CI: 0.54-0.72) after internal-external cross-validation. Calibration varied across studies.The prediction model for recurrent CAT included five clinical predictors and has only modest discrimination. Prediction of recurrent CAT at the initiation of anticoagulation remains challenging.
The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/).
Conflict of interest statement
M.G. is an employee of Daiichi Sankyo. A.Y.Y.L. reports consulting fees and honoraria from Bayer AG, consulting fees and honoraria from LEO Pharma, consulting fees and honoraria from Pfizer, consulting fees from Servier, and honoraria from Bristol Myers Squibb. M.D.N. reports personal fees as an invited speaker from Bayer, Daiichi Sankyo, and Viatris, personal fees for advisory board membership from Leo Pharma, Janssen, and Pfizer, and institutional funding from Leo Pharma, all outside the submitted work. G.E.R. reports consultancy fees or honoraria from AMAG Pharma, Alnylam, Anthos Therapeutics, Bayer HealthCare Pharmaceuticals Inc., Bristol-Myers Squibb, Daiichi Sankyo Inc., Ionis, Janssen Global Services LLC, Pfizer, Regeneron, Sirius Pharmaceutical; honoraria from BMS, Pfizer, Daiichi Sankyo; DSMB or advisory board membership from Anthos Therapeutics, Janssen, Bristol-Myers Squibb, and Pfizer, leadership or fiduciary role in other board, society, committee or advocacy group of OU Health, and the National Blood Clot Alliance. P.W.K. reports research funding from Daiichi Sankyo and Roche diagnostics. H.R.B. reports research support from Sanofi-Aventis, Bayer HealthCare, Bristol-Myers Squibb, Daiichi-Sankyo, GlaxoSmithKline, Pfizer, Roche, IONIS, Boehringer Ingelheim, Eli Lilly, and Novartis. Consultant from Sanofi-Aventis, Bayer HealthCare, Bristol-Myers Squibb, Daiichi-Sankyo, GlaxoSmithKline, Pfizer, Roche, IONIS, Boehringer Ingelheim, Eli Lilly, and Novartis. Scientific advisory board from Sanofi-Aventis, Bayer HealthCare, Bristol-Myers Squibb, Daiichi-Sankyo, GlaxoSmithKline, Pfizer, Roche, IONIS, Boehringer Ingelheim, Eli Lilly, and Novartis. N.v.E. reports advisory board honoraria from Daiichi Sankyo, LEO Pharma, and Bayer, which were transferred to his institute. The other authors have nothing to declare.
Figures
References
-
- Timp J F, Braekkan S K, Versteeg H H, Cannegieter S C. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122(10):1712–1723. - PubMed
-
- Hokusai VTE Cancer Investigators . Raskob G E, van Es N, Verhamme P et al.Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378(07):615–624. - PubMed
-
- Young A M, Marshall A, Thirlwall J et al.Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D) J Clin Oncol. 2018;36(20):2017–2023. - PubMed
-
- McBane R D, II, Wysokinski W E, Le-Rademacher J G et al.Apixaban and dalteparin in active malignancy-associated venous thromboembolism: the ADAM VTE trial. J Thromb Haemost. 2020;18(02):411–421. - PubMed
-
- Caravaggio Investigators . Agnelli G, Becattini C, Meyer G et al.Apixaban for the treatment of venous thromboembolism associated with cancer. N Engl J Med. 2020;382(17):1599–1607. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous