Timing of oral anticoagulation in atrial fibrillation patients after acute ischaemic stroke and outcome after 3 months: results of the multicentre Berlin Atrial Fibrillation Registry
- PMID: 39299734
- PMCID: PMC11428999
- DOI: 10.1136/openhrt-2024-002688
Timing of oral anticoagulation in atrial fibrillation patients after acute ischaemic stroke and outcome after 3 months: results of the multicentre Berlin Atrial Fibrillation Registry
Abstract
Background: Oral anticoagulation (OAC) is key in stroke prevention in patients with atrial fibrillation (AF) but there is uncertainty regarding the optimal timing of OAC (re)initiation after stroke, as recent large randomised controlled trials have methodological weaknesses and excluded stroke patients on therapeutic anticoagulation at stroke onset as well as patients started on a vitamin K antagonist after stroke. The '1-3-6-12 days rule', based on expert consensus and referring to stroke severity, was used in clinical practice to initiate OAC after acute ischaemic stroke or transient ischaemic attack (TIA) since publication in 2013.
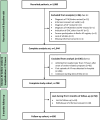
Methods: We retrospectively assessed whether compliance to the '1-3-6-12 days rule' was associated with the composite endpoint (recurrent stroke, systemic embolism, myocardial infarction, major bleeding or all-cause death).
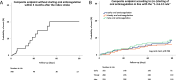
Results: Among 708 registry patients with known AF before stroke and hospitalisation within 72 hours after stroke, 432 were anticoagulated at stroke onset. OAC was started according to the '1-3-6-12 days rule' in 255 (39.2%) patients. Non-adherence to the '1-3-6-12 days rule' was not associated with the composite endpoint within 3 months in 661 patients who (re-)started on OAC (log-rank test: p=0.74).Results were similar for 521 patients (re)started on a non-vitamin K-dependent OAC.
Conclusion: (Re)starting OAC after stroke followed the '1-3-6-12 days rule' in about 40% of all patients with AF, and more often in those anticoagulated at stroke onset. Adherence to the '1-3-6-12 days rule' did not reduce the composite clinical endpoint, if OAC was restarted within 3 months of stroke/TIA.
Trial registration number: NCT02306824.
Keywords: Arrhythmias, Cardiac; Atrial Fibrillation; STROKE.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: DGN reports speaker's honoraria and/or consulting fees from AstraZeneca, Bayer Healthcare, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Novartis, Pfizer and Sanofi. PUH reports grants from Charité – Universitätsmedizin Berlin (within Mondafis; supported by an unrestricted research grant to the Charité from Bayer), research grants from German Ministry of Research and Education, German Research Foundation, research grants from Bavarian State, European Union, German Parkinson Society, German Cancer Aid, University Hospital Würzburg, German Heart Foundation, Federal Joint Committee (G-BA) within the Innovationsfonds, University Hospital Heidelberg (within RASUNOA-prime; supported by an unrestricted research grant to the University Hospital Heidelberg from Bayer, BMS, Boehringer-Ingelheim, Daiichi Sankyo), University Göttingen (within FIND-AF randomised; supported by an unrestricted research grant to the University Göttingen from Boehringer-Ingelheim) outside the submitted work; and participated on Data, Safety Monitoring Board in publicly funded studies (by German Research Foundation, German Ministry of Research, Foundations). ME reports grants from Bayer and fees paid to the Charité from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers-Squibb, Covidien, Daiichi Sankyo, Glaxo Smith Kline, Novartis, Pfizer and Sanofi,all outside the submitted work. KGH reports speaker's honoraria, consulting fees, lecture honoraria and/or study grants from Abbott, Amarin; AstraZeneca, Bayer Healthcare, Biotronik, Boehringer Ingelheim, Boston Scientific, Bristol-Myers Squibb, Daiichi Sankyo, Edwards Lifesciences, Medronic, Novartis, Pfizer, Portola, Premier Research, Sanofi, SUN Pharma, and W.L. Gore and Associates. PS reports speaker's honoraria from Bayer, Boehringer Ingelheim, Bristol-Myers-Squibb and Pfizer.
Figures
References
-
- Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): The Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612. - DOI - PubMed
-
- Joglar JA, Chung MK, Armbruster AL, et al. 2023 ACC/AHA/ACCP/HRS Guideline for the Diagnosis and Management of Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2024;149:e1–156. doi: 10.1161/CIR.0000000000001193. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
