Attainment of EULAR/ERA-EDTA targets of therapy with current immunosuppressive regimens and adjustments in treatment: a multicentre, real-life observational study
- PMID: 39299738
- PMCID: PMC11429000
- DOI: 10.1136/rmdopen-2024-004437
Attainment of EULAR/ERA-EDTA targets of therapy with current immunosuppressive regimens and adjustments in treatment: a multicentre, real-life observational study
Abstract
Objective: To estimate real-life European Alliance of Associations for Rheumatology (EULAR)/European Renal Association (ERA)-European Dialysis and Transplantation Association (EDTA) response rates and predictors for no response in patients with lupus nephritis (LN) managed with conventional immunosuppressive therapies.
Methods: Ambidirectional cohort study of patients with new-onset LN (period 2014-to date). Response rates in the first year were calculated, and all treatment modifications were recorded. Univariate and multivariate regression analyses were performed to assess determinants of failure to respond at 12 months.
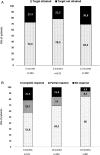
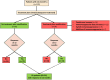
Results: 140 patients were included (81.4% women, median (IQR) age at LN diagnosis 38 (22) years). Among them, 32.1% presented with nephrotic range proteinuria, 28.6% with glomerular filtration rate <60 mL/min, 76.6% had proliferative and 19.7% class V LN. Initial treatment consisted of cyclophosphamide in 51.4% of patients (84.7% high-dose, 15.3% low-dose) and mycophenolate in 32.1%. 120 patients had available data at 12 months. EULAR/ERA-EDTA renal response rates at 3, 6 and 12 months were achieved by 72.6%, 78.5% % and 69.2% of patients, respectively. In multivariate analysis, increased Chronicity Index at baseline was associated with failure to achieve either complete or partial response at 12 months (OR 2.26, 95% CI 1.35 to 3.77). Notably, 20% of patients required treatment modifications due to suboptimal response during the first 12 months, with the addition of or switch to a different immunosuppressive drug in seven and nine patients, respectively.
Conclusions: More than two-thirds of patients with LN attain EULAR/ERA-EDTA response rates by 12 months, but 20% require therapy modifications within this time period. Patients with increased chronicity in baseline biopsy, when combined with histological activity, are at higher risk for a lack of clinical response.
Keywords: Cyclophosphamide; Lupus Erythematosus, Systemic; Lupus Nephritis.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
