Differential Encoding of Two-Tone Harmonics in the Male and Female Mouse Auditory Cortex
- PMID: 39299802
- PMCID: PMC11529816
- DOI: 10.1523/JNEUROSCI.0364-24.2024
Differential Encoding of Two-Tone Harmonics in the Male and Female Mouse Auditory Cortex
Abstract
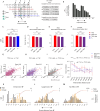
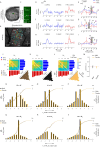
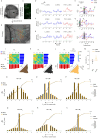
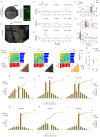
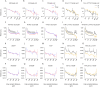
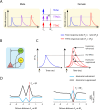
Harmonics are an integral part of music, speech, and vocalizations of animals. Since the rest of the auditory environment is primarily made up of nonharmonic sounds, the auditory system needs to perceptually separate the above two kinds of sounds. In mice, harmonics, generally with two-tone components (two-tone harmonic complexes, TTHCs), form an important component of vocal communication. Communication by pups during isolation from the mother and by adult males during courtship elicits typical behaviors in female mice-dams and adult courting females, respectively. Our study shows that the processing of TTHC is specialized in mice providing neural basis for perceptual differences between tones and TTHCs and also nonharmonic sounds. Investigation of responses in the primary auditory cortex (Au1) from in vivo extracellular recordings and two-photon Ca2+ imaging of excitatory and inhibitory neurons to TTHCs exhibit enhancement, suppression, or no-effect with respect to tones. Irrespective of neuron type, harmonic enhancement is maximized, and suppression is minimized when the fundamental frequencies (F 0) match the neuron's best fundamental frequency (BF0). Sex-specific processing of TTHC is evident from differences in the distributions of neurons' best frequency (BF) and best fundamental frequency (BF0) in single units, differences in harmonic suppressed cases re-BF0, independent of neuron types, and from pairwise noise correlations among excitatory and parvalbumin inhibitory interneurons. Furthermore, TTHCs elicit a higher response compared with two-tone nonharmonics in females, but not in males. Thus, our study shows specialized neural processing of TTHCs over tones and nonharmonics, highlighting local network specialization among different neuronal types.
Keywords: auditory cortex; excitatory neurons; inhibitory neurons; sex specificity; two-tone harmonic complex; vocalization.
Copyright © 2024 the authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

References
MeSH terms
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
