MFSD7C protects hemolysis-induced lung impairments by inhibiting ferroptosis
- PMID: 39300060
- PMCID: PMC11413235
- DOI: 10.1038/s41467-024-52537-6
MFSD7C protects hemolysis-induced lung impairments by inhibiting ferroptosis
Abstract
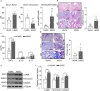
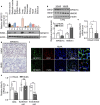
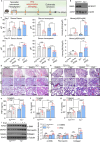
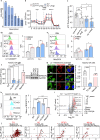
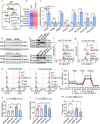
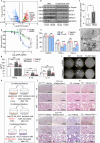
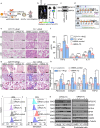
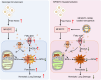
Hemolysis drives susceptibility to lung injury and predicts poor outcomes in diseases, such as malaria and sickle cell disease (SCD). However, the underlying pathological mechanism remains elusive. Here, we report that major facilitator superfamily domain containing 7 C (MFSD7C) protects the lung from hemolytic-induced damage by preventing ferroptosis. Mechanistically, MFSD7C deficiency in HuLEC-5A cells leads to mitochondrial dysfunction, lipid remodeling and dysregulation of ACSL4 and GPX4, thereby enhancing lipid peroxidation and promoting ferroptosis. Furthermore, systemic administration of MFSD7C mRNA-loaded nanoparticles effectively prevents lung injury in hemolytic mice, such as HbSS-Townes mice and PHZ-challenged 7 C-/- mice. These findings present the detailed link between hemolytic complications and ferroptosis, providing potential therapeutic targets for patients with hemolytic disorders.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Lamikanra, A. A. et al. Malarial anemia: of mice and men. Blood110, 18–28 (2007). - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

