Metastable water at several compression rates and its freezing kinetics into ice VII
- PMID: 39300088
- PMCID: PMC11413240
- DOI: 10.1038/s41467-024-52576-z
Metastable water at several compression rates and its freezing kinetics into ice VII
Abstract
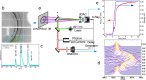
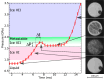
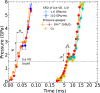
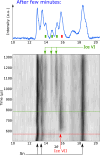
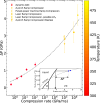
Water can be dynamically over-compressed well into the stability field of ice VII. Whether water then transforms into ice VII, vitreous ice or a metastable novel crystalline phase remained uncertain. We report here the freezing of over-compressed water to ice VII by time-resolved X-ray diffraction. Quasi-isothermal dynamic compression paths are achieved using a dynamic-piezo-Diamond-Anvil-Cell, with programmable pressure rise time from 0.1 ms to 100 ms. By combining the present data set with those obtained on various ns-dynamical platforms, a complete evolution of the solidification pressure of metastable water versus the compression rate is rationalized within the classical nucleation theory framework. Also, when crystallization into ice VII occurs in between 1.6 GPa and 2.0 GPa, that is in the stability field of ice VI, a structural evolution over few ms is then observed into a mixture of ice VI and ice VII that seems to resolve apparent contradictions between previous results.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Petrenko, V. F. & Whitworth, R. W. Physics of Ice (Oxford University Press, 1999).
-
- Bartels-Rausch, T. et al. Ice structures, patterns, and processes: a view across the icefields. Rev. Mod. Phys.84, 885–944 (2012).
LinkOut - more resources
Full Text Sources
Miscellaneous

