Sequential immunization with chimeric hemagglutinin ΔNS1 attenuated influenza vaccines induces broad humoral and cellular immunity
- PMID: 39300090
- PMCID: PMC11413010
- DOI: 10.1038/s41541-024-00952-7
Sequential immunization with chimeric hemagglutinin ΔNS1 attenuated influenza vaccines induces broad humoral and cellular immunity
Abstract
Influenza viruses pose a threat to public health as evidenced by severe morbidity and mortality in humans on a yearly basis. Given the constant changes in the viral glycoproteins owing to antigenic drift, seasonal influenza vaccines need to be updated periodically and effectiveness often drops due to mismatches between vaccine and circulating strains. In addition, seasonal influenza vaccines are not protective against antigenically shifted influenza viruses with pandemic potential. Here, we have developed a highly immunogenic vaccination regimen based on live-attenuated influenza vaccines (LAIVs) comprised of an attenuated virus backbone lacking non-structural protein 1 (ΔNS1), the primary host interferon antagonist of influenza viruses, with chimeric hemagglutinins (cHA) composed of exotic avian head domains with a highly conserved stalk domain, to redirect the humoral response towards the HA stalk. In this study, we showed that cHA-LAIV vaccines induce robust serum and mucosal responses against group 1 stalk and confer antibody-dependent cell cytotoxicity activity. Mice that intranasally received cH8/1-ΔNS1 followed by a cH11/1-ΔNS1 heterologous booster had robust humoral responses for influenza A virus group 1 HAs and were protected from seasonal H1N1 influenza virus and heterologous highly pathogenic avian H5N1 lethal challenges. When compared with mice immunized with the standard of care or cold-adapted cHA-LAIV, cHA-ΔNS1 immunized mice had robust antigen-specific CD8+ T-cell responses which also correlated with markedly reduced lung pathology post-challenge. These observations support the development of a trivalent universal influenza vaccine for the protection against group 1 and group 2 influenza A viruses and influenza B viruses.
© 2024. The Author(s).
Conflict of interest statement
The A.G.-S. laboratory has received research support from GSK, Pfizer, Senhwa Biosciences, Kenall Manufacturing, Blade Therapeutics, Avimex, Johnson & Johnson, Dynavax, 7Hills Pharma, Pharmamar, ImmunityBio, Accurius, Nanocomposix, Hexamer, N-fold LLC, Model Medicines, Atea Pharma, Applied Biological Laboratories and Merck. A.G.-S. has consulting agreements for the following companies involving cash and/or stock: Castlevax, Amovir, Vivaldi Biosciences, Contrafect, 7Hills Pharma, Avimex, Pagoda, Accurius, Esperovax, Farmak, Applied Biological Laboratories, Pharmamar, CureLab Oncology, CureLab Veterinary, Synairgen, Paratus, Pfizer and Prosetta. A.G.-S. has been an invited speaker in meeting events organized by Seqirus, Janssen, Abbott and AstraZeneca. A.G.-S. and P.P. are inventors on patents and patent applications on the use of antivirals and vaccines for the treatment and prevention of virus infections and cancer, owned by the Icahn School of Medicine at Mount Sinai, New York. The M.S. laboratory has received unrelated funding support in sponsored research agreements from Phio Pharmaceuticals, 7Hills Pharma, ArgenX, and Moderna. The Icahn School of Medicine at Mount Sinai has filed patent applications regarding influenza virus vaccines on which F.K. is listed as inventor. The Krammer laboratory has received support for influenza virus research in the past from GSK and is currently receiving support from Dynavax. The Icahn School of Medicine at Mount Sinai has filed patent applications relating to influenza virus vaccines and therapeutics vaccines, which lists F.K. as co-inventor. Several of these patents have been licensed and F.K. has received royalty payments from commercial entities. F.K. has consulted from Merck, Pfizar, Seqirus, GSK and Curevac and is currently consulting for Gritstone, 3rd Rock Ventures and Avimex and he is co-founder and scientific advisory board member of Castlevax. The F.K. laboratory is also collaborating with Dynavax on influenza virus vaccine development and with VIR on influenza therapeutics. T.M. is inventor on patents and patent applications on vaccines and immunotherapies, owned by Vivaldi Biosciences and BlueSky Immunotherapies. T.M. owns stocks and options from BlueSky Immunotherapies, Nuvonis Technologies and Vivaldi Biosciences. A.A. is inventor on patents and patent applications on vaccines and immunotherapies, owned by Vivaldi Biosciences and BlueSky Immunotherapies. A.A. owns stock options from Vivaldi Biosciences. All other authors declare no competing interests.
Figures


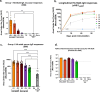

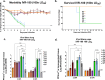





References
-
- Krammer, F. The human antibody response to influenza A virus infection and vaccination. Nat. Rev. Immunol.19, 383–397 (2019). - PubMed
Grants and funding
- R01 AI141226/AI/NIAID NIH HHS/United States
- R21 AI176069/AI/NIAID NIH HHS/United States
- R21AI151229/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- S10 OD030269/OD/NIH HHS/United States
- R21 AI151229/AI/NIAID NIH HHS/United States
- P01 AI097092/AI/NIAID NIH HHS/United States
- 75N93021C00014/AI/NIAID NIH HHS/United States
- 75N93019C00051/AI/NIAID NIH HHS/United States
- R01AI141226/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- G20 AI174733/AI/NIAID NIH HHS/United States
- G20AI174733/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- S10 OD026983/OD/NIH HHS/United States
- P01AI097092/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R21AI176069/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

