Disrupted basolateral amygdala circuits supports negative valence bias in depressive states
- PMID: 39300117
- PMCID: PMC11412998
- DOI: 10.1038/s41398-024-03085-6
Disrupted basolateral amygdala circuits supports negative valence bias in depressive states
Abstract
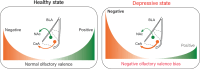
Negative bias is an essential characteristic of depressive episodes leading patients to attribute more negative valence to environmental cues. This negative bias affects all levels of information processing including emotional response, attention and memory, leading to the development and maintenance of depressive symptoms. In this context, pleasant stimuli become less attractive and unpleasant ones more aversive, yet the related neural circuits underlying this bias remain largely unknown. By studying a mice model for depression chronically receiving corticosterone (CORT), we showed a negative bias in valence attribution to olfactory stimuli that responds to antidepressant drug. This result paralleled the alterations in odor value assignment we observed in bipolar depressed patients. Given the crucial role of amygdala in valence coding and its strong link with depression, we hypothesized that basolateral amygdala (BLA) circuits alterations might support negative shift associated with depressive states. Contrary to humans, where limits in spatial resolution of imaging tools impair easy amygdala segmentation, recently unravelled specific BLA circuits implicated in negative and positive valence attribution could be studied in mice. Combining CTB and rabies-based tracing with ex vivo measurements of neuronal activity, we demonstrated that negative valence bias is supported by disrupted activity of specific BLA circuits during depressive states. Chronic CORT administration induced decreased recruitment of BLA-to-NAc neurons preferentially involved in positive valence encoding, while increasing recruitment of BLA-to-CeA neurons preferentially involved in negative valence encoding. Importantly, this dysfunction was dampened by chemogenetic hyperactivation of BLA-to-NAc neurons. Moreover, altered BLA activity correlated with durable presynaptic connectivity changes coming from the paraventricular nucleus of the thalamus, recently demonstrated as orchestrating valence assignment in the amygdala. Together, our findings suggest that specific BLA circuits alterations might support negative bias in depressive states and provide new avenues for translational research to understand the mechanisms underlying depression and treatment efficacy.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Leppänen JM. Emotional information processing in mood disorders: a review of behavioral and neuroimaging findings. Curr Opin Psychiatry. 2006;19:34–9. - PubMed
-
- Clark L, Chamberlain SR, Sahakian BJ. Neurocognitive Mechanisms in Depression: Implications for Treatment. Annu Rev Neurosci. 2009;32:57–74. - PubMed
-
- Harmer CJ, O’Sullivan U, Favaron E, Massey-Chase R, Ayres R, Reinecke A, et al. Effect of Acute Antidepressant Administration on Negative Affective Bias in Depressed Patients. AJP. 2009;166:1178–84. - PubMed
-
- Bigot M, Alonso M, Houenou J, Sarrazin S, Dargél AA, Lledo P-M, et al. An emotional-response model of bipolar disorders integrating recent findings on amygdala circuits. Neurosci Biobehav Rev. 2020;118:358–66. - PubMed
MeSH terms
Substances
Grants and funding
- ANR-15-NEUC-0004-02/Agence Nationale de la Recherche (French National Research Agency)
- ANR-AAPG2021/Agence Nationale de la Recherche (French National Research Agency)
- ANR-10-LABX-73/Agence Nationale de la Recherche (French National Research Agency)
- ANR-AAPG2021/Agence Nationale de la Recherche (French National Research Agency)
- ANR-15-NEUC-0004-02/Agence Nationale de la Recherche (French National Research Agency)
LinkOut - more resources
Full Text Sources

