The gut microbiota and its metabolite butyrate shape metabolism and antiviral immunity along the gut-lung axis in the chicken
- PMID: 39300162
- PMCID: PMC11413219
- DOI: 10.1038/s42003-024-06815-0
The gut microbiota and its metabolite butyrate shape metabolism and antiviral immunity along the gut-lung axis in the chicken
Abstract
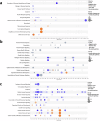
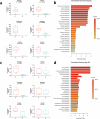
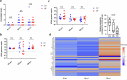
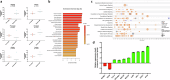
The gut microbiota exerts profound influence on poultry immunity and metabolism through mechanisms that yet need to be elucidated. Here we used conventional and germ-free chickens to explore the influence of the gut microbiota on transcriptomic and metabolic signatures along the gut-lung axis in poultry. Our results demonstrated a differential regulation of certain metabolites and genes associated with innate immunity and metabolism in peripheral tissues of germ-free birds. Furthermore, we evidenced the gut microbiota's capacity to regulate mucosal immunity in the chicken lung during avian influenza virus infection. Finally, by fine-analysing the antiviral pathways triggered by the short-chain fatty acid (SCFA) butyrate in chicken respiratory epithelial cells, we found that it regulates interferon-stimulated genes (ISGs), notably OASL, via the transcription factor Sp1. These findings emphasize the pivotal role of the gut microbiota and its metabolites in shaping homeostasis and immunity in poultry, offering crucial insights into the mechanisms governing the communication between the gut and lungs in birds.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Thaiss, C. A., Zmora, N., Levy, M. & Elinav, E. The microbiome and innate immunity. Nature535, 65–74 (2016). - PubMed
-
- Willing, B. P., Russell, S. L. & Finlay, B. B. Shifting the balance: antibiotic effects on host–microbiota mutualism. Nat. Rev. Microbiol.9, 233–243 (2011). - PubMed
-
- Koh, A., De Vadder, F., Kovatcheva-Datchary, P. & Bäckhed, F. From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cell165, 1332–1345 (2016). - PubMed
-
- Fachi, J. L. et al. Butyrate protects mice from clostridium difficile-induced colitis through an HIF-1-dependent mechanism. Cell Rep.27, 750–761.e7 (2019). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

