Impact of BCR::ABL1 single nucleotide variants on asciminib efficacy
- PMID: 39300220
- PMCID: PMC11518997
- DOI: 10.1038/s41375-024-02411-7
Impact of BCR::ABL1 single nucleotide variants on asciminib efficacy
Abstract
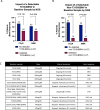
Asciminib is a potent and selective inhibitor of BCR::ABL1, with potential to avoid toxicity resulting from off-target kinase inhibition. Forty-nine patients treated with asciminib under a managed access program in the UK were evaluated for toxicity and response. Intolerance, rather than resistance (65% vs. 35%), was the most common reason for cessation of the last-line of treatment but asciminib was well tolerated, with most patients (29, 59%) remaining on treatment at a median of 14 months follow-up, and only 6 (12%) stopping for intolerance. Of 44 patients assessable for response, 29 (66%) achieved a complete cytogenetic response (CCyR) or better, with poorer responses seen in those stopping their last-line of therapy for resistance. Fewer patients with a prior history of a non-T315I-BCR::ABL1 single nucleotide variant (BSNV), or a non-T315I-BSNV detectable at baseline achieved CCyR. Serial tracking of BSNV by next generation sequencing demonstrated clonal expansion of BSNV-harbouring populations, which in some settings was associated with resistance (E459K, F317L, F359I), while in others was seen in the context of ongoing response, often with intensified dosing (T315I, I502F). These data suggest that asciminib exerts selective pressure on some BSNV-harbouring populations in vivo, some of which may respond to intensified dosing.
© 2024. The Author(s).
Conflict of interest statement
AJI: Speakers bureau (Incyte), speakers bureau and advisory board (Novartis). JB: Speaker fees and advisory board (Novartis and Incyte). MC: Research funding (Cyclacel and Incyte), advisory board member (Novartis, Incyte, Jazz Pharmaceuticals, Pfizer and Servier), honoraria (Astellas, Novartis). RF: Honoraria (Novartis, BMS), advisory board (CTI biopharma and GSK). GHa: Honoraria and Speakers fees (Novartis, Pfizer, Incyte), Advisory board (Novartis), DA: Speaker fees (Incyte). JFA: Honoraria, research funding, and speakers bureau (Incyte, Pfizer); honoraria and speakers bureau (Bristol Myers Squibb, Novartis). DM: Honoraria (Incyte, Novartis, Pfizer, Ascentage Pharma), Research funding (Incyte and Pfizer). The remaining authors (CH VO, SC FF AK, DR, PG, SF, GHo, MR, CA, AC, TC, NC, AD, PF, PG, SH, BJPH, JH, SM, KR, JK) report no conflict of interest.
Figures



References
-
- Wylie AA, Schoepfer J, Jahnke W, Cowan-Jacob SW, Loo A, Furet P, et al. The allosteric inhibitor ABL001 enables dual targeting of BCR-ABL1. Nature 2017;543:733–7. - PubMed
-
- Bower H, Björkholm M, Dickman PW, Höglund M, Lambert PC, Andersson TM. Life expectancy of patients with chronic myeloid leukemia approaches the life expectancy of the general population. J Clin Oncol 2016;34:2851–7. - PubMed
-
- Lipton JH, Brümmendorf TH, Gambacorti-Passerini C, Garcia-Gutiérrez V, Deininger MW, Cortes JE. Long-term safety review of tyrosine kinase inhibitors in chronic myeloid leukemia - What to look for when treatment-free remission is not an option. Blood Rev 2022;56:100968. - PubMed
-
- Fabarius A, Giehl M, Rebacz B, Krämer A, Frank O, Haferlach C, et al. Centrosome aberrations and G1 phase arrest after in vitro and in vivo treatment with the SRC/ABL inhibitor dasatinib. Haematologica 2008;93:1145–54. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

