A tRNA modification with aminovaleramide facilitates AUA decoding in protein synthesis
- PMID: 39300229
- PMCID: PMC11938285
- DOI: 10.1038/s41589-024-01726-x
A tRNA modification with aminovaleramide facilitates AUA decoding in protein synthesis
Abstract
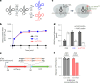
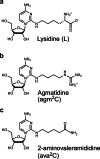
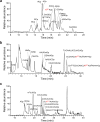
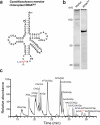
Modified tRNA anticodons are critical for proper mRNA translation during protein synthesis. It is generally thought that almost all bacterial tRNAsIle use a modified cytidine-lysidine (L)-at the first position (34) of the anticodon to decipher the AUA codon as isoleucine (Ile). Here we report that tRNAsIle from plant organelles and a subset of bacteria contain a new cytidine derivative, designated 2-aminovaleramididine (ava2C). Like L34, ava2C34 governs both Ile-charging ability and AUA decoding. Cryo-electron microscopy structural analyses revealed molecular details of codon recognition by ava2C34 with a specific interaction between its terminal amide group and an mRNA residue 3'-adjacent to the AUA codon. These findings reveal the evolutionary variation of an essential tRNA modification and demonstrate the molecular basis of AUA decoding mediated by a unique tRNA modification.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: All authors declare no competing interests.
Figures







References
-
- Suzuki, T. The expanding world of tRNA modifications and their disease relevance. Nat. Rev. Mol. Cell Biol.22, 375–392 (2021). - PubMed
-
- Grosjean, H. (ed.). Fine-Tuning of RNA Functions by Modification and Editing Vol. 12, pp. 23–69 (Springer-Verlag GmbH, 2005).
-
- Ohira, T. & Suzuki, T. Transfer RNA modifications and cellular thermotolerance. Mol. Cell84, 94–106 (2024). - PubMed
-
- Helm, M. & Motorin, Y. Detecting RNA modifications in the epitranscriptome: predict and validate. Nat. Rev. Genet.18, 275–291 (2017). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 13J09842/MEXT | Japan Society for the Promotion of Science (JSPS)
- R01 AI042347/AI/NIAID NIH HHS/United States
- JP223fa627001/Japan Agency for Medical Research and Development (AMED)
- 26113003/MEXT | Japan Society for the Promotion of Science (JSPS)
- R37 AI042347/AI/NIAID NIH HHS/United States
- AI-042347/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- JPMJER2002/MEXT | JST | Exploratory Research for Advanced Technology (ERATO)
- 26220205/MEXT | Japan Society for the Promotion of Science (JSPS)
- 22K06075/MEXT | Japan Society for the Promotion of Science (JSPS)
- 23KJ0409/MEXT | Japan Society for the Promotion of Science (JSPS)
- 18H05272/MEXT | Japan Society for the Promotion of Science (JSPS)
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

