Liver protects neuron viability and electrocortical activity in post-cardiac arrest brain injury
- PMID: 39300235
- PMCID: PMC11479250
- DOI: 10.1038/s44321-024-00140-z
Liver protects neuron viability and electrocortical activity in post-cardiac arrest brain injury
Abstract
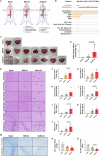
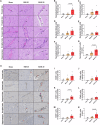
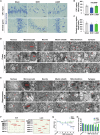
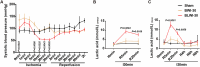
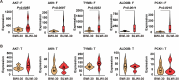
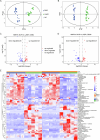
Brain injury is the leading cause of mortality among patients who survive cardiac arrest (CA). Clinical studies have shown that the presence of post-CA hypoxic hepatitis or pre-CA liver disease is associated with increased mortality and inferior neurological recovery. In our in vivo global cerebral ischemia model, we observed a larger infarct area, elevated tissue injury scores, and increased intravascular CD45+ cell adhesion in reperfused brains with simultaneous hepatic ischemia than in those without it. In the ex vivo brain normothermic machine perfusion (NMP) model, we demonstrated that addition of a functioning liver to the brain NMP circuit significantly reduced post-CA brain injury, increased neuronal viability, and improved electrocortical activity. Furthermore, significant alterations were observed in both the transcriptome and metabolome in the presence or absence of hepatic ischemia. Our study highlights the crucial role of the liver in the pathogenesis of post-CA brain injury.
Keywords: Brain Injury; Cardiac Arrest; Ketone Body Production; Liver Dysfunction; Normothermic Machine Perfusion.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Al-Khatib (2018) 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm 15:E276–E277 - PubMed
-
- Atwood C, Eisenberg MS, Herlitz J, Rea TD (2005) Incidence of EMS-treated out-of-hospital cardiac arrest in Europe. Resuscitation 67:75–80 - PubMed
-
- Berdowski J, Berg RA, Tijssen JGP, Koster RW (2010) Global incidences of out-of-hospital cardiac arrest and survival rates: Systematic review of 67 prospective studies. Resuscitation 81:1479–1487 - PubMed
MeSH terms
Grants and funding
- 81970564,82170663,82370664/MOST | National Natural Science Foundation of China (NSFC)
- 82070670/MOST | National Natural Science Foundation of China (NSFC)
- 82300744/MOST | National Natural Science Foundation of China (NSFC)
- 2023B1212060020/Guangdong Provincial Key Laboratory of Construction Projection on Organ Donation and Transplant Immunology
- 2020A0505020003/Guangdong Provincial International Cooperation Base of Science and Technology (Organ Transplantation)
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

