The potential of circulating microRNAs as novel diagnostic biomarkers of COVID-19: a systematic review and meta-analysis
- PMID: 39300343
- PMCID: PMC11414062
- DOI: 10.1186/s12879-024-09915-8
The potential of circulating microRNAs as novel diagnostic biomarkers of COVID-19: a systematic review and meta-analysis
Abstract
Introduction: The COVID-19 pandemic has caused an unprecedented health threat globally, necessitating innovative and efficient diagnostic approaches for timely identification of infected individuals. Despite few emerging reports, the clinical utility of circulating microRNAs (miRNAs) in early and accurate diagnosis of COVID-19 is not well-evidenced. Hence, this meta-analysis aimed to explore the diagnostic potential of circulating miRNAs for COVID-19. The protocol for this study was officially recorded on PROSPERO under registration number CRD42023494959.
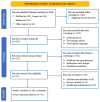
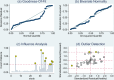
Methods: Electronic databases including Embase, PubMed, Scopus, and other sources were exhaustively searched to recover studies published until 16th January, 2024. Pooled specificity, sensitivity, positive likelihood ratio (PLR), negative likelihood ratio (NLR), diagnostic ratio (DOR), positive predictive value (PPV), negative predictive value (NPV), and area under the curve (AUC) were computed from the metadata using Stata 14.0 software. Risk of bias appraisal of included articles was carried out using Review Manager (Rev-Man) 5.3 package through the modified QUADAS-2 tool. Subgroup, heterogeneity, meta-regression and sensitivity analyses were undertaken. Publication bias and clinical applicability were also evaluated via Deeks' funnel plot and Fagan nomogram (scattergram), respectively.
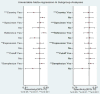
Result: A total of 43 studies from 13 eligible articles, involving 5175 participants (3281 COVID-19 patients and 1894 healthy controls), were analyzed. Our results depicted that miRNAs exhibit enhanced pooled specificity 0.91 (95% CI: 0.88-0.94), sensitivity 0.94 (95% CI: 0.91-0.96), DOR of 159 (95% CI: 87-288), and AUC values of 0.97 (95% CI: 0.95-0.98) with high pooled PPV 96% (95% CI: 94-97%) and NPV 88% (95% CI: 86-90%) values. Additionally, highest diagnostic capacity was observed in studies involving larger sample size (greater than 100) and those involving the African population, demonstrating consistent diagnostic effectiveness across various specimen types. Notably, a total of 12 distinct miRNAs were identified as suitable for both exclusion and confirmation of COVID-19 cases, denoting their potential clinical applicability.
Conclusion: Our study depicted that miRNAs show significantly high diagnostic accuracy in differentiating COVID-19 patients from healthy counterparts, suggesting their possible use as viable biomarkers. Nonetheless, thorough and wide-ranging longitudinal researches are necessary to confirm the clinical applicability of miRNAs in diagnosing COVID-19.
Keywords: COVID-19; Circulating miRNAs; Diagnostic biomarker; Meta-analysis; Systematic review.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- World Health Organization. Coronavirus (COVID–19) Dashboard. [Internet]. 2024 [cited 16 January 2024]. https://data.who.int/dashboards/covid19/deaths?n=c
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

