Phase IB part of LOC-R01, a LOC network non-comparative randomized phase IB/II study testing R-MPV in combination with escalating doses of lenalidomide or ibrutinib for newly diagnosed primary central nervous system lymphoma (PCNSL) patients
- PMID: 39300447
- PMCID: PMC11414093
- DOI: 10.1186/s13045-024-01606-w
Phase IB part of LOC-R01, a LOC network non-comparative randomized phase IB/II study testing R-MPV in combination with escalating doses of lenalidomide or ibrutinib for newly diagnosed primary central nervous system lymphoma (PCNSL) patients
Erratum in
-
Correction: Phase IB part of LOC-R01, a LOC network non-comparative randomized phase IB/II study testing R-MPV in combination with escalating doses of lenalidomide or ibrutinib for newly diagnosed primary central nervous system lymphoma (PCNSL) patients.J Hematol Oncol. 2024 Oct 19;17(1):98. doi: 10.1186/s13045-024-01620-y. J Hematol Oncol. 2024. PMID: 39427203 Free PMC article. No abstract available.
Abstract
Background: Results of conventional induction chemotherapies in primary central nervous system lymphoma (PCNSL) need to be improved. Ibrutinib, a BTK inhibitor, and lenalidomide, an immunomodulatory drug, have shown promising results at relapse, supporting to further assess their individual use in combination with high-dose methotrexate-based chemotherapy.
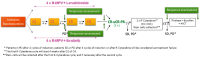
Methods: Patients with newly diagnosed PCNSL were randomized to receive four 28-day cycles of ibrutinib or lenalidomide in combination with R-MPV (rituximab, methotrexate, procarbazine, vincristine and prednisone) in a 3 + 3 design. Responders then received a consolidation with R-Cytarabine and an intensive chemotherapy with autologous stem cell transplantation. The objective of the phase IB study was to define the recommended phase II dose (RP2D) based on the dose-limiting toxicity (DLT) occurring during the first induction cycle.
Results: Twenty-six patients (median age 52) were randomized. Four DLTs were observed: one grade 5 aspergillosis and pneumocystosis, one grade 4 catheter-related infection and two grade 3 increased alanine aminotransferase levels. RP2D of ibrutinib and lenalidomide were 560 mg daily (D3-14 and D17-28) and 15 mg daily (D1-21) respectively, in combination with R-MPV. In both arms, the most frequent grade ≥3 treatment-related adverse events were hepatic cytolysis, neutropenia and infections. One grade 4 Lyell's syndrome was reported at cycle 2 in the lenalidomide arm. After 4 induction cycles, the overall response rates were 76.9% and 83.3% in the lenalidomide and ibrutinib arm, respectively.
Conclusion: Targeted induction therapies combining lenalidomide or ibrutinib with R-MPV are feasible for first-line PCNSL. The safety profile is consistent with the known safety profiles of R-MPV and both targeted therapies. The phase II part of the study is ongoing.
Trial registration: NCT04446962.
Keywords: Ibrutinib; Lenalidomide; Phase IB trial; Primary central nervous system lymphoma.
© 2024. The Author(s).
Conflict of interest statement
AM performed scientific and medical consulting for Novartis, Janssen, Kite/Gilead and MSD and received research grants from Mnemo Therapeutics. OL has received honoraria and travel fundings from Roche, Kite/Gilead, BMS, Janssen and AstraZeneca. HR has received honoraria from Kite/Gilead, Novartis, Incyte, Janssen, MSD, Takeda and Roche; and is a member on an entity’s Board of Directors or advisory committees of Kite/Gilead, Novartis, Bristol-Myers Squibb/Celgene, ADC Therapeutics, Incyte and Miltenyi. SC received a research grant from AstraZeneca.
Figures


References
-
- Hernández-Verdin I, Kirasic E, Wienand K, Mokhtari K, Eimer S, Loiseau H et al. Molecular and clinical diversity in primary central nervous system lymphoma. Annals Oncol 2023 Feb;34(2):186-199. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

