The chemical composition of secondary organic aerosols regulates transcriptomic and metabolomic signaling in an epithelial-endothelial in vitro coculture
- PMID: 39300536
- PMCID: PMC11411994
- DOI: 10.1186/s12989-024-00600-x
The chemical composition of secondary organic aerosols regulates transcriptomic and metabolomic signaling in an epithelial-endothelial in vitro coculture
Abstract
Background: The formation of secondary organic aerosols (SOA) by atmospheric oxidation reactions substantially contributes to the burden of fine particulate matter (PM2.5), which has been associated with adverse health effects (e.g., cardiovascular diseases). However, the molecular and cellular effects of atmospheric aging on aerosol toxicity have not been fully elucidated, especially in model systems that enable cell-to-cell signaling.
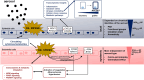
Methods: In this study, we aimed to elucidate the complexity of atmospheric aerosol toxicology by exposing a coculture model system consisting of an alveolar (A549) and an endothelial (EA.hy926) cell line seeded in a 3D orientation at the air‒liquid interface for 4 h to model aerosols. Simulation of atmospheric aging was performed on volatile biogenic (β-pinene) or anthropogenic (naphthalene) precursors of SOA condensing on soot particles. The similar physical properties for both SOA, but distinct differences in chemical composition (e.g., aromatic compounds, oxidation state, unsaturated carbonyls) enabled to determine specifically induced toxic effects of SOA.
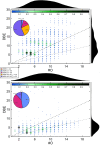
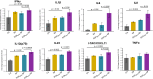
Results: In A549 cells, exposure to naphthalene-derived SOA induced stress-related airway remodeling and an early type I immune response to a greater extent. Transcriptomic analysis of EA.hy926 cells not directly exposed to aerosol and integration with metabolome data indicated generalized systemic effects resulting from the activation of early response genes and the involvement of cardiovascular disease (CVD) -related pathways, such as the intracellular signal transduction pathway (PI3K/AKT) and pathways associated with endothelial dysfunction (iNOS; PDGF). Greater induction following anthropogenic SOA exposure might be causative for the observed secondary genotoxicity.
Conclusion: Our findings revealed that the specific effects of SOA on directly exposed epithelial cells are highly dependent on the chemical identity, whereas non directly exposed endothelial cells exhibit more generalized systemic effects with the activation of early stress response genes and the involvement of CVD-related pathways. However, a greater correlation was made between the exposure to the anthropogenic SOA compared to the biogenic SOA. In summary, our study highlights the importance of chemical aerosol composition and the use of cell systems with cell-to-cell interplay on toxicological outcomes.
Keywords: Airway remodeling; Endothelial dysfunction; Epithelial-endothelial coculture; Inflammation; Secondary organic aerosols (SOA).
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Brook RD, Rajagopalan S, Pope CA 3rd, Brook JR, Bhatnagar A, Diez-Roux AV, et al. Particulate matter air pollution and cardiovascular disease: an update to the scientific statement from the American Heart Association. Circulation. 2010;121(21):2331–78. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

