Clinical-grade intranasal NGF fuels neurological and metabolic functions of Mecp2-deficient mice
- PMID: 39300821
- PMCID: PMC11884770
- DOI: 10.1093/brain/awae291
Clinical-grade intranasal NGF fuels neurological and metabolic functions of Mecp2-deficient mice
Abstract
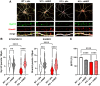
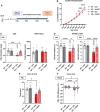
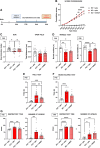
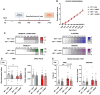
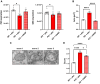
MECP2 deficiency causes a broad spectrum of neuropsychiatric disorders that can affect both genders. Rett syndrome is the most common and is characterized by an apparently normal growth period followed by a regression phase in which patients lose most of their previously acquired skills. After this dramatic period, various symptoms progressively appear, including severe intellectual disability, epilepsy, apraxia, breathing abnormalities and motor deterioration. MECP2 encodes for an epigenetic transcription factor that is particularly abundant in the brain; consequently, several transcriptional defects characterize the Rett syndrome brain. The well-known deficiency of several neurotrophins and growth factors, together with the positive effects exerted by trofinetide, a synthetic analogue of insulin-like growth factor 1, in Rett patients and in mouse models of Mecp2 deficiency, prompted us to investigate the therapeutic potential of nerve growth factor. Initial in vitro studies demonstrated a healing effect of recombinant human GMP-grade NGF (rhNGF) on neuronal maturation and activity in cultured Mecp2-null neurons. Subsequently, we designed in vivo studies with clear translational potential using intranasally administered rhNGF already used in the clinic. The efficacy of rhNGF in vivo in Mecp2-null hemizygous male mice and heterozygous female mice was assessed. General well-being was evaluated by a conventional phenotypic score and motor performance through the Pole and Beam Walking tests, while cognitive function and interaction with the environment were measured by the Novel Object Recognition test and the Marble Burying test, respectively. At the end of the treatment, mouse cortices were dissected and bulk RNA sequencing was performed to identify the molecular pathways involved in the protective effects of rhNGF. In both male and female mouse models of Rett syndrome, rhNGF exerted positive effects on cognitive and motor functions. In male hemizygous mice, which suffer from significantly more severe and rapidly advancing symptoms, the drug's ability to slow the disease's progression was more pronounced. The unbiased research for the molecular mechanisms triggering the observed benefits revealed a strong positive effect on gene sets related to oxidative phosphorylation, mitochondrial structure and function. These results were validated by demonstrating the drug's ability to improve mitochondrial structure and respiration in Mecp2-null cerebral cortices. Furthermore, Gene Ontology analyses indicated that NGF exerted the expected improvement in neuronal maturation. We conclude that intranasal administration of rhNGF is a non-invasive and effective route of administration for the treatment of Rett syndrome and possibly for other neurometabolic disorders with overt mitochondrial dysfunction.
Keywords: Mecp2 null mice; Rett syndrome; animal behaviour; cognitive function; mitochondrial dysfunction; rhNGF.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
The authors report no competing interests.
Figures


References
-
- Frasca A, Kilstrup-Nielsen C, Landsberger N. Rett syndrome: From the involved gene(s) to treatment. In: Zigmond MJ, Wiley CA, Chesselet M-F, eds. Neurobiology of brain disorders. 2nd edn. Academic Press; 2023:89–113.
-
- Chahrour M, Zoghbi HY. The story of Rett syndrome: From clinic to neurobiology. Neuron. 2007;56:422–437. - PubMed
-
- Guy J, Hendrich B, Holmes M, Martin JE, Bird A. A mouse Mecp2-null mutation causes neurological symptoms that mimic Rett syndrome. Nat Genet. 2001;27:322–326. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

