Comparable Results Between 8 and 12 Gray TBI in Combination With Fludarabine and Post-Transplant Cyclophosphamide in MRD-Negative but Not in MRD-Positive Acute Lymphoblastic Leukemia Patients Transplanted in First Complete Remission
- PMID: 39300830
- PMCID: PMC11613535
- DOI: 10.1111/ejh.14305
Comparable Results Between 8 and 12 Gray TBI in Combination With Fludarabine and Post-Transplant Cyclophosphamide in MRD-Negative but Not in MRD-Positive Acute Lymphoblastic Leukemia Patients Transplanted in First Complete Remission
Abstract
Background: The optimal TBI dose for ALL patients undergoing allogeneic SCT is still not clearly defined.
Methods: Single-center retrospective analysis of high-risk ALL patients in CR1 treated with 8 Gy (n = 22) or 12 Gy (n = 50) TBI in combination with fludarabine and PTCy. Median patient age in the 8 Gy TBI cohort was 63 (37-79) and 37 (18-56) in the 12 Gy TBI cohort and median follow-up time was 21 months (range 1-92).
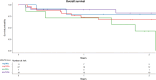
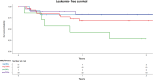
Results: OS and LFS at 2 years after 8 Gy were 65% and 55% versus 74% and 74% after 12 Gy (p = 0.3 and p = 0.2, respectively). CIR and NRM at 2 years were 27% and 14% after 8 Gy versus 4% and 20% after 12 Gy (p = 0.004 and p = 0.4, respectively). MRD-positive (+) patients (n = 26) receiving 12 Gy (n = 19) showed better OS (p = 0.01), LFS (p = 0.009), GRFS, lower CIR (p = 0.02), and similar NRM than did MRD+ patients receiving 8 Gy (n = 7). MRD-negative (-) patients (n = 38) receiving 12 Gy (n = 27) had similar OS, LFS, GRFS, lower CIR, and higher NRM (p = 0.04) than did MRD- patients receiving 8 Gy (n = 11).
Conclusion: Our study demonstrates that 8 Gy TBI in comparison to 12 Gy TBI results in low NRM but a high relapse rate with similar OS, LFS, and GRFS. In MRD+ high-risk ALL patients, allogeneic SCT with 12 Gy TBI leads to improved OS, LFS, GRFS, and a low relapse rate. Prospective studies comparing the different treatment regimens with larger MRD patient cohorts are needed to confirm this data.
Keywords: 12 Gy TBI; 8 Gy TBI; PTCy; acute lymphoblastic leukemia; allogeneic stem cell transplantation.
© 2024 The Author(s). European Journal of Haematology published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Kantarjian H., Thomas D., O'Brien S., et al., “Long‐Term Follow‐Up Results of Hyperfractionated Cyclophosphamide, Vincristine, Doxorubicin, and Dexamethasone (Hyper‐CVAD), a Dose‐Intensive Regimen, in Adult Acute Lymphocytic Leukemia,” Cancer 101, no. 12 (2004): 2788–2801. - PubMed
-
- Gökbuget N., Kneba M., Raff T., et al., “Adult Patients With Acute Lymphoblastic Leukemia and Molecular Failure Display a Poor Prognosis and Are Candidates for Stem Cell Transplantation and Targeted Therapies,” Blood 120, no. 9 (2012): 1868–1876. - PubMed
-
- Champlin R., Khouri I., Shimoni A., et al., “Harnessing Graft‐Versus‐Malignancy: Non‐Myeloablative Preparative Regimens for Allogeneic Haematopoietic Transplantation, an Evolving Strategy for Adoptive Immunotherapy,” British Journal of Haematology 111, no. 1 (2000): 18–29. - PubMed
-
- Giebel S., Labopin M., Socié G., et al., “Improving Results of Allogeneic Hematopoietic Cell Transplantation for Adults With Acute Lymphoblastic Leukemia in First Complete Remission: An Analysis From the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation,” Haematologica 102, no. 1 (2017): 139–149. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

