Viscoelasticity of Hyaluronic Acid Hydrogels Regulates Human Pluripotent Stem Cell-derived Spinal Cord Organoid Patterning and Vascularization
- PMID: 39300854
- PMCID: PMC11671291
- DOI: 10.1002/adhm.202402199
Viscoelasticity of Hyaluronic Acid Hydrogels Regulates Human Pluripotent Stem Cell-derived Spinal Cord Organoid Patterning and Vascularization
Abstract
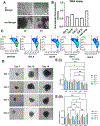
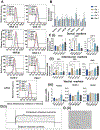
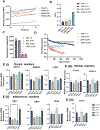
Recently, it has been recognized that natural extracellular matrix (ECM) and tissues are viscoelastic, while only elastic properties have been investigated in the past. How the viscoelastic matrix regulates stem cell patterning is critical for cell-ECM mechano-transduction. Here, this study fabricated different methacrylated hyaluronic acid (HA) hydrogels using covalent cross-linking, consisting of two gels with similar elasticity (stiffness) but different viscoelasticity, and two gels with similar viscoelasticity but different elasticity (stiffness). Meanwhile, a second set of dual network hydrogels are fabricated containing both covalent and coordinated cross-links. Human spinal cord organoid (hSCO) patterning in HA hydrogels and co-culture with isogenic human blood vessel organoids (hBVOs) are investigated. The viscoelastic hydrogels promote regional hSCO patterning compared to the elastic hydrogels. More viscoelastic hydrogels can promote dorsal marker expression, while softer hydrogels result in higher interneuron marker expression. The effects of viscoelastic properties of the hydrogels become more dominant than the stiffness effects in the co-culture of hSCOs and hBVOs. In addition, more viscoelastic hydrogels can lead to more Yes-associated protein nuclear translocation, revealing the mechanism of cell-ECM mechano-transduction. This research provides insights into viscoelastic behaviors of the hydrogels during human organoid patterning with ECM-mimicking in vitro microenvironments for applications in regenerative medicine.
Keywords: human pluripotent stem cells; hyaluronic acid hydrogels; spinal cord organoid patterning; vascularization, viscoelasticity.
© 2024 Wiley‐VCH GmbH.
Conflict of interest statement
Competing interests:
Authors declare that they have no competing interests.
Figures




References
-
- D’Mello R, Dickenson AH, Spinal cord mechanisms of pain, British journal of anaesthesia 101(1) (2008) 8–16. - PubMed
-
- Revah O, Gore F, Kelley KW, Andersen J, Sakai N, Chen X, Li MY, Birey F, Yang X, Saw NL, Baker SW, Amin ND, Kulkarni S, Mudipalli R, Cui B, Nishino S, Grant GA, Knowles JK, Shamloo M, Huguenard JR, Deisseroth K, Pasca SP, Maturation and circuit integration of transplanted human cortical organoids, Nature 610(7931) (2022) 319–326. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

