Targeting the cancer cells and cancer-associated fibroblasts with next-generation FGFR inhibitors in prostate cancer co-culture models
- PMID: 39300962
- PMCID: PMC11413502
- DOI: 10.1002/cam4.70240
Targeting the cancer cells and cancer-associated fibroblasts with next-generation FGFR inhibitors in prostate cancer co-culture models
Abstract
Background: Inhibition of androgen receptor (AR) signaling is the main treatment strategy in advanced prostate cancer (PCa). A subset of castration resistant prostate cancer (CRPC) bypasses the AR blockade by increased fibroblast growth factor receptor (FGFR) signaling. The first- and second-generation, non-covalent FGFR inhibitors (FGFRis) have largely failed in the clinical trials against PCa.
Purpose: In this study, we tested the drug sensitivity of LNCaP, VCaP, and CWR-R1PCa cell lines to second-generation, covalent FGFRis (FIIN1, FIIN2) and a novel FGFR downstream molecule inhibitor (FRS2αi).
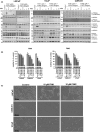
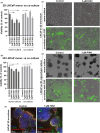
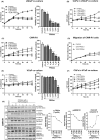
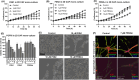
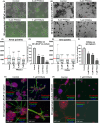
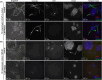
Methods: 2D and 3D mono- and co-cultures of cancer cells, and cancer-associated fibroblasts (CAFs) were used to mimic tumor-stroma interactions in the extracellular matrix (ECM). The treatment responses of the FGFR signaling molecules, the viability and proliferation of cancer cells, and CAFs were determined through immunoblotting, migration assay, cell viability assay, and real-time imaging. Immunofluorescent and confocal microscopy images of control and treated cultures of cancer cells and CAFs, and their morphometric data were deduced.
Results: The FGFRis were more effective in mono-cultures of the cancer cells compared with co-cultures with CAFs. The FRS2αi was specifically effective in co-cultures with CAFs but was not cytotoxic to CAF mono-cultures as in the case of FIIN1 and FIIN2. At the molecular level, FRS2αi decreased p-FRS2α, p-ERK1/2, and activated apoptosis as monitored by cleaved caspase-3 activity in a concentration-dependent manner in the co-cultures. We observed no synergistic drug efficacy in the combination treatment of the FGFRi with ARi, enzalutamide, and darolutamide. The FRS2αi treatment led to a decrease in proliferation of cancer cell clusters in co-cultures as indicated by their reduced size and Ki67 expression.
Conclusions: CAFs exert a protective effect on cancer cells and should be included in the in vitro models to make them physiologically more relevant in screening and testing of FGFRis. The FRS2αi was the most potent agent in reducing the viability and proliferation of the 3D organotypic co-cultures, mainly by disrupting the contact between CAFs and cancer cell clusters. The next-generation FGFRi, FRS2αi, may be a better alternative treatment option for overcoming ARi treatment resistance in advanced PCa.
Keywords: AR antagonist; FGFR inhibitors; FRS2α inhibitor; cancer‐associated fibroblasts; castration resistant prostate cancer; darolutamide; enzalutamide.
© 2024 The Author(s). Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
References
MeSH terms
Substances
Grants and funding
- the Drug Research Doctoral Program of the University of Turku, Finland
- 2022043/the Finnish-Norwegian Medical Foundation/Suomalais-Norjalainen Lääketieteen Säätiö
- the Paulo Foundation/Paulon Säätiö, Finland
- the Southwest Finland Cancer Organization/Lounaissuomalaiset Syöpäjärjestöt
- 080956/Turku University Foundation/Turun Yliopistosäätiö
- 721746/the European Union's TransPot Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement
- 267326/Research Council of Finland
- 309372/Research Council of Finland
- KFS-4853-08-2019/Swiss Cancer Research Foundation
- SNF_310030_188793/Schweizerischer Nationalfonds zur Förderung der Wissenschaftlichen Forschung
- 20230339/Ida Montinin Säätiö
- University of Turku/Turun Yliopisto
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

