Pentadentate and Hexadentate Pyridinophane Ligands Support Reversible Cu(II)/Cu(I) Redox Couples
- PMID: 39301085
- PMCID: PMC11412068
- DOI: 10.3390/inorganics11110446
Pentadentate and Hexadentate Pyridinophane Ligands Support Reversible Cu(II)/Cu(I) Redox Couples
Abstract
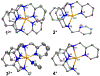
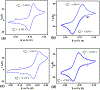
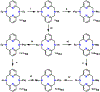
Two new ligands were synthesized with the goal of copper stabilization, N,N'-(2-methylpyridine)-2,11-diaza[3,3](2,6)pyridinophane (PicN4) and N-(methyl),N'-(2-methylpyridine)-2,11-diaza[3,3](2,6)pyridinophane (PicMeN4), by selective functionalization of HN4 and TsHN4. These two ligands, when reacted with various copper salts, generated both Cu(II) and Cu(I) complexes. These ligands and Cu complexes were characterized by various methods, such as NMR, UV-Vis, MS, and EA. Each compound was also examined electrochemically, and each revealed reversible Cu(II)/Cu(I) redox couples. Additionally, stability constants were determined via spectrophotometric titrations, and radiolabeling and cytotoxicity experiments were performed to assess the chelators relevance to their potential use in vivo as 64Cu PET imaging agents.
Keywords: 64Cu PET imaging agents; bioinorganic chemistry; copper(I) complexes; copper(II) complexes; cyclic voltammetry; pyridinophane ligands; radiolabeling; reversibility.
Conflict of interest statement
Conflicts of Interest: The authors declare no competing financial interest.
Figures
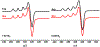




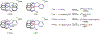
References
-
- Krylova K; Kulatilleke CP; Heeg MJ; Salhi CA; Ochrymowycz LA; Rorabacher DB A Structural Strategy for Generating Rapid Electron-Transfer Kinetics in Copper(II/I) Systems. Inorg. Chem 1999, 38, 4322.
-
- Rorabacher DB Electron Transfer by Copper Centers. Chem. Rev 2004, 104, 651. - PubMed
-
- Mirica LM; Ottenwaelder X; Stack TDP Structure and Spectroscopy of Copper-Dioxygen Complexes. Chem. Rev 2004, 104, 1013. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
