Synthetic pathways to create asymmetric center at C1 position of 1-substituted-tetrahydro-β-carbolines - a review
- PMID: 39301229
- PMCID: PMC11411349
- DOI: 10.1039/d4ra05961a
Synthetic pathways to create asymmetric center at C1 position of 1-substituted-tetrahydro-β-carbolines - a review
Abstract
The 2,3,4,9-tetrahydro-1H-pyrido[3,4-b]indoles or tetrahydro-β-carbolines (THβCs) are tricyclic compounds that are found in various natural sources that exhibit a wide range of important pharmacological activities. Chiral 1-substituted-THβCs, which have an asymmetric center at C1, have attained significant interest due to their possible Monoamine Oxidase (MAO) inhibitory activity, benzodiazepine receptor binding activity, and antimalarial effectiveness against chloroquine-resistant Plasmodium falciparum. This review highlights and summarizes various novel stereoselective approaches to introduce chirality at the C1 position of 1-substituted-THβCs in good yield and enantiomeric excess (ee) or diastereomeric excess (de). These methods include the Pictet-Spengler reaction, chiral auxiliary, Asymmetric Transfer Hydrogenation (ATH) with chiral catalysts, asymmetric addition reaction, and enzymatic catalysis. The syntheses of chiral THβCs are reviewed comprehensively, emphasizing their role in drug development from 1977 to 2024.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






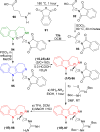
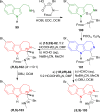
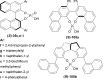


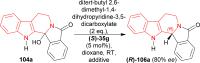









References
-
- Hesse M., Alkaloids: Nature's Curse or Blessing?, WILEY-VCH, Weinheim, 2002
-
- Daugan A. Grondin P. Ruault C. Le Monnier de Gouville A.-C. Coste H. Linget J. M. Kirilovsky J. Hyafil F. Labaudinière R. J. Med. Chem. 2003;46:4533–4542. - PubMed
-
- Hotha S. Yarrow J. C. Yang J. G. Garrett S. Renduchintala K. V. Mayer T. U. Kapoor T. M. Angew. Chem., Int. Ed. 2003;42:2379–2382. - PubMed
-
- Van Maarseveen J. H. Hermkens P. H. H. De Clercq E. Balzarini J. Scheeren H. W. Kruse C. G. J. Med. Chem. 1992;35:3223–3230. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

