Cu-doped ZnO/Ag/CuO heterostructure: superior photocatalysis and charge transfer
- PMID: 39301231
- PMCID: PMC11409720
- DOI: 10.1039/d4ra05989a
Cu-doped ZnO/Ag/CuO heterostructure: superior photocatalysis and charge transfer
Abstract
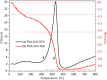
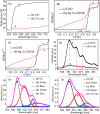
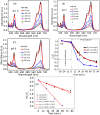
Doped semiconductor heterostructures have superior properties compared to their components. In this study, we observed the synthesis of Cu-doped ZnO/Ag/CuO heterostructure with the presence of charge transfer and visible light-harvesting properties resulting from doping and heterojunction. The porous heterostructures were prepared using the bottom-up combustion (BUC) approach. This method generated porous heterostructures by eliminating gaseous by-products. X-ray diffraction (XRD) optimization revealed that the ideal conditions included 1.00 g of polyvinyl alcohol (PVA), a synthesis temperature of 50 °C, and a 1 hour calcination time. Introducing copper (Cu) into the zinc oxide (ZnO) lattice caused a high-angle shift in the XRD pattern peaks. High-resolution transmission electron microscopy (HRTEM) images and XRD patterns confirmed the formation of Cu-doped ZnO/Ag/CuO (c-zac) heterostructures. Elemental mapping analysis confirmed the even surface distribution of Ag metal. The c-zac heterostructures exhibited superior optoelectrical and charge transfer properties compared to single ZnO. The heterostructures demonstrated improved methylene blue (MB) dye degradation potential (k = 0.065 min-1) compared to single ZnO (k = 0.0041 min-1). This photocatalytic potential is attributed to enhanced light absorption and charge transfer properties. The extended visible light absorption resulted from CuO and Ag's surface plasmon resonance properties. The selected 15c-zac heterostructure also performed well in a reusability photocatalytic test, remaining effective until the 3rd cycle. Consequently, this heterostructure holds promise for scaling up as a catalyst for environmental remediation.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures






References
-
- Oladoye P. O. Ajiboye T. O. Omotola E. O. Oyewola O. J. Results Eng. 2022;16:100678. doi: 10.1016/j.rineng.2022.100678. - DOI
-
- Hu F. Yu D. Ye M. Wang H. Hao Y. Wang L. Li L. Han X. Peng S. Adv. Energy Mater. 2022;12:1–10.
-
- Sun X. Li L. Jin S. Shao W. Wang H. Zhang X. Xie Y. eScience. 2023;3:100095. doi: 10.1016/j.esci.2023.100095. - DOI
-
- Hao Y. Hu F. Zhu S. Sun Y. Wang H. Wang L. Wang Y. Xue J. Liao Y. Shao M. Peng S. Angew. Chem., Int. Ed. 2023;62:1–8. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

