Intervention in gut microbiota increases intestinal γ-aminobutyric acid and alleviates anxiety behavior: a possible mechanism via the action on intestinal epithelial cells
- PMID: 39301289
- PMCID: PMC11410766
- DOI: 10.3389/fcimb.2024.1421791
Intervention in gut microbiota increases intestinal γ-aminobutyric acid and alleviates anxiety behavior: a possible mechanism via the action on intestinal epithelial cells
Abstract
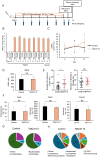
The role of the gut microbiota in the gut-brain axis has attracted attention in recent years. Some gut microbiota produces γ-aminobutyric acid (GABA), a major inhibitory neurotransmitter in mammals, in vitro, but the correlation between gut microbiota composition and intestinal GABA concentration, as well as the action of intestinal GABA in vivo, are poorly understood. Herein, we found that the intestinal GABA concentration was increased in mice by the intervention of the gut microbiota with neomycin or Bifidobacterium bifidum TMC3115 (TMC3115). Administration of TMC3115 reduced anxiety without affecting serum levels of serotonin, corticosterone, or GABA. We further found that intestinal epithelial cells expressed GABA receptor subunits and mediated mitogen-activated protein kinase signaling upon GABA stimulation. In addition, administration of TMC3115 induced mitogen-activated protein kinase signaling in colonic epithelial cells but not in small intestinal epithelial cells in mice. These results indicate that GABA produced by the gut microbiota, mainly in the colon, may affect host behavioral characteristics via GABA receptors expressed in intestinal epithelial cells without being transferred to the blood. This study suggests a novel mechanism by which intestinal GABA exerts physiological effects, even in the presence of the blood-brain barrier.
Keywords: gut microbiota; gut-brain axis; intestinal epithelial cell; probiotics; γ-aminobutyric acid.
Copyright © 2024 Ikegami, Narabayashi, Nakata, Yamashita, Sugi, Fuji, Matsufuji, Harata, Yoda, Miyazawa, Nakanishi and Takahashi.
Conflict of interest statement
GH, KY, and KM are employees of Takanashi Milk Products Co., Ltd. Kanagawa, Japan. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

