The lysogenic filamentous Pseudomonas bacteriophage phage Pf slows mucociliary transport
- PMID: 39301510
- PMCID: PMC11412248
- DOI: 10.1093/pnasnexus/pgae390
The lysogenic filamentous Pseudomonas bacteriophage phage Pf slows mucociliary transport
Abstract
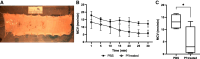
Pseudomonas aeruginosa is a major pulmonary pathogen causing chronic pulmonary infections in people with cystic fibrosis (CF). The P. aeruginosa filamentous and lysogenic bacteriophage, Pf phage, is abundant in the airways of many people with CF and has been associated with poor outcomes in a cross-sectional cohort study. Previous studies have identified roles for Pf phage in biofilm formation, specifically forming higher-order birefringent, liquid crystals when in contact with other biopolymers in biofilms. Liquid crystalline biofilms are more adherent and viscous than those without liquid crystals. A key feature of biofilms is to enhance bacterial adherence and resist physical clearance. The effect of Pf phage on mucociliary transport is unknown. We found that primary CF and non-CF nasal epithelial cells cultured at air-liquid interface treated with Pf phage exhibit liquid crystalline structures in the overlying mucus. On these cell cultures, Pf phage entangles cilia but does not affect ciliary beat frequency. In both these in vitro cell cultures and in an ex vivo porcine trachea model, introduction of Pf phage decreases mucociliary transport velocity. Pf phage also blocks the rescue of mucociliary transport by CF transmembrane conductance regulator modulators in CF cultures. Thus, Pf phage may contribute to the pathogenesis of P. aeruginosa-associated CF lung disease via induction of liquid crystalline characteristics to airway secretions, leading to impaired mucociliary transport. Targeting Pf phage may be useful in treatment CF as well as other settings of chronic P. aeruginosa infections.
Keywords: bacteriophage; cystic fibrosis; mucociliary transport.
© The Author(s) 2024. Published by Oxford University Press on behalf of National Academy of Sciences.
Figures



References
-
- Khan T, et al. . 1995. Early pulmonary inflammation in infants with cystic-fibrosis. Am J Respir Crit Care Med. 151(4):1075–1082. - PubMed
-
- Van Goor F, et al. . 2006. Rescue of ΔF508-CFTR trafficking and gating in human cystic fibrosis airway primary cultures by small molecules. Am J Physiol Lung Cell Mol Physiol. 290(6):L1117–L1130. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

