Donor-derived Cell-free DNA Evaluation in Pediatric Heart Transplant Recipients: A Single-center 12-mo Experience
- PMID: 39301559
- PMCID: PMC11410329
- DOI: 10.1097/TXD.0000000000001689
Donor-derived Cell-free DNA Evaluation in Pediatric Heart Transplant Recipients: A Single-center 12-mo Experience
Abstract
Background: Endomyocardial biopsy (EMB) is considered the gold-standard method to diagnose rejection after heart transplantation. However, the many disadvantages and potential complications of this test restrict its routine application, particularly in pediatric patients. Donor-derived cell-free DNA (dd-cfDNA), released by the transplanted heart as result of cellular injury, is emerging as a biomarker of tissue damage involved in ischemia/reperfusion injury and posttransplant rejection. In the present study, we systematically evaluated dd-cfDNA levels in pediatric heart transplant patients coming for follow-up visits to our clinic for 12 mo, with the aim of determining whether dd-cfDNA monitoring could be efficiently applied and integrated into the posttransplant management of rejection in pediatric recipients.
Methods: Twenty-nine patients were enrolled, and cfDNA was obtained from 158 blood samples collected during posttransplant follow-up. dd-cfDNA% was determined with a droplet-digital polymerase chain reaction assay. EMB scores, donor-specific antibody measurements, and distress marker quantification were correlated with dd-cfDNA, together with echocardiogram information.
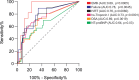
Results: The percentage of dd-cfDNA increased when EMBs scored positive for rejection (P = 0.0002) and donor-specific antibodies were present (P = 0.0010). N-terminal pro-B-type natriuretic peptide and high-sensitive troponin I elevation were significantly associated with dd-cfDNA release (P = 0.02 and P < 0.0001, respectively), as were reduced isovolumetric relaxation time (P = 0.0031), signs of heart failure (P = 0.0018), and treatment for rejection (P = 0.0017). By determining a positive threshold for rejection at 0.55%, the test had a negative predictive value maximized at 100%.
Conclusions: Collectively, results indicate that dd-cfDNA monitoring has a high negative prognostic value, suggesting that in heart transplanted children with dd-cfDNA levels of <0.55% threshold, protocol EMBs may be postponed.
Copyright © 2024 The Author(s). Transplantation Direct. Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Bleiweis MS, Fricker FJ, Upchurch GR, et al. . Heart transplantation in patients less than 18 years of age: comparison of 2 eras over 36 years and 323 transplants at a single institution. J Am Coll Surg. 2023;236:898–909. - PubMed
-
- Scott JP, Ragalie WS, Stamm KD, et al. . Total cell-free DNA predicts death and infection following pediatric and adult heart transplantation. Ann Thorac Surg. 2021;112:1282–1289. - PubMed
-
- Oellerich M, Sherwood K, Keown P, et al. . Liquid biopsies: donor-derived cell-free DNA for the detection of kidney allograft injury. Nat Rev Nephrol. 2021;17:591–603. - PubMed
LinkOut - more resources
Full Text Sources

