A role for leucine-rich, glioma inactivated 1 in regulating pain sensitivity
- PMID: 39301592
- PMCID: PMC11884686
- DOI: 10.1093/brain/awae302
A role for leucine-rich, glioma inactivated 1 in regulating pain sensitivity
Abstract
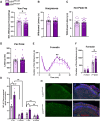
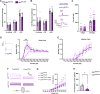
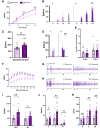
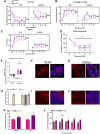
Neuronal hyperexcitability is a key driver of persistent pain states, including neuropathic pain. Leucine-rich, glioma inactivated 1 (LGI1) is a secreted protein known to regulate excitability within the nervous system and is the target of autoantibodies from neuropathic pain patients. Therapies that block or reduce antibody levels are effective at relieving pain in these patients, suggesting that LGI1 has an important role in clinical pain. Here we have investigated the role of LGI1 in regulating neuronal excitability and pain-related sensitivity by studying the consequences of genetic ablation in specific neuron populations using transgenic mouse models. LGI1 has been well studied at the level of the brain, but its actions in the spinal cord and peripheral nervous system are poorly understood. We show that LGI1 is highly expressed in dorsal root ganglion (DRG) and spinal cord dorsal horn neurons in both mouse and human. Using transgenic mouse models, we genetically ablated LGI1, either specifically in nociceptors (LGI1fl/Nav1.8+) or in both DRG and spinal neurons (LGI1fl/Hoxb8+). On acute pain assays, we found that loss of LGI1 resulted in mild thermal and mechanical pain-related hypersensitivity when compared with littermate controls. In LGI1fl/Hoxb8+ mice, we found loss of Kv1 currents and hyperexcitability of DRG neurons. LGI1fl/Hoxb8+ mice displayed a significant increase in nocifensive behaviours in the second phase of the formalin test (not observed in LGI1fl/Nav1.8+ mice), and extracellular recordings in LGI1fl/Hoxb8+ mice revealed hyperexcitability in spinal dorsal horn neurons, including enhanced wind-up. Using the spared nerve injury model, we found that LGI1 expression was dysregulated in the spinal cord. LGI1fl/Nav1.8+ mice showed no differences in nerve injury-induced mechanical hypersensitivity, brush-evoked allodynia or spontaneous pain behaviour compared with controls. However, LGI1fl/Hoxb8+ mice showed a significant exacerbation of mechanical hypersensitivity and allodynia. Our findings point to effects of LGI1 at the level of both the DRG and the spinal cord, including an important impact of spinal LGI1 on pathological pain. Overall, we find a novel role for LGI1 with relevance to clinical pain.
Keywords: hyperexcitability; leucine-rich, glioma inactivated 1 (LGI1); mechanical pain hypersensitivity; neuropathic pain; wind-up.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Guarantors of Brain.
Conflict of interest statement
No competing interests.
Figures

References
-
- Van Hecke O, Austin SK, Khan RA, Smith BH, Torrance N. Neuropathic pain in the general population: A systematic review of epidemiological studies. Pain. 2014;155:654–662. - PubMed
-
- Serra J, Bostock H, Solà R, et al. Microneurographic identification of spontaneous activity in C-nociceptors in neuropathic pain states in humans and rats. Pain. 2012;153:42–55. - PubMed
-
- Djouhri L, Zeidan A, Alzoghaibi M, Al Otaibi MF, Abd El-Aleem SA. L5 spinal nerve axotomy induces distinct electrophysiological changes in axotomized L5- and adjacent L4-dorsal root ganglion neurons in rats in vivo. J Neurotrauma. 2021;38:330–341. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

