Identification and Potential Clinical Utility of Common Genetic Variants in Gestational Diabetes among Chinese Pregnant Women
- PMID: 39301664
- PMCID: PMC11788552
- DOI: 10.4093/dmj.2024.0139
Identification and Potential Clinical Utility of Common Genetic Variants in Gestational Diabetes among Chinese Pregnant Women
Abstract
Backgruound: The genetic basis for hyperglycaemia in pregnancy remain unclear. This study aimed to uncover the genetic determinants of gestational diabetes mellitus (GDM) and investigate their applications.
Methods: We performed a meta-analysis of genome-wide association studies (GWAS) for GDM in Chinese women (464 cases and 1,217 controls), followed by de novo replications in an independent Chinese cohort (564 cases and 572 controls) and in silico replication in European (12,332 cases and 131,109 controls) and multi-ethnic populations (5,485 cases and 347,856 controls). A polygenic risk score (PRS) was derived based on the identified variants.
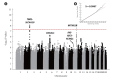
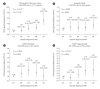
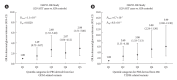
Results: Using the genome-wide scan and candidate gene approaches, we identified four susceptibility loci for GDM. These included three previously reported loci for GDM and type 2 diabetes mellitus (T2DM) at MTNR1B (rs7945617, odds ratio [OR], 1.64; 95% confidence interval [CI], 1.38 to 1.96), CDKAL1 (rs7754840, OR, 1.33; 95% CI, 1.13 to 1.58), and INS-IGF2-KCNQ1 (rs2237897, OR, 1.48; 95% CI, 1.23 to 1.79), as well as a novel genome-wide significant locus near TBR1-SLC4A10 (rs117781972, OR, 2.05; 95% CI, 1.61 to 2.62; Pmeta=7.6×10-9), which has not been previously reported in GWAS for T2DM or glycaemic traits. Moreover, we found that women with a high PRS (top quintile) had over threefold (95% CI, 2.30 to 4.09; Pmeta=3.1×10-14) and 71% (95% CI, 1.08 to 2.71; P=0.0220) higher risk for GDM and abnormal glucose tolerance post-pregnancy, respectively, compared to other individuals.
Conclusion: Our results indicate that the genetic architecture of glucose metabolism exhibits both similarities and differences between the pregnant and non-pregnant states. Integrating genetic information can facilitate identification of pregnant women at a higher risk of developing GDM or later diabetes.
Keywords: Diabetes, gestational; Genetic risk score; Genome-wide association study; Glucose intolerance; Pregnant women.
Conflict of interest statement
Cadmon King-poo Lim, Juliana Chung-ngor Chan, and Ronald Ching-wan Ma are co-founders of GemVCare, a technology start-up initiated with support from the Hong Kong Government Innovation and Technology Commission and its Technology Start-up Support Scheme for Universities (TSSSU). The other authors declare that there is no duality of interest associated with this manuscript. Ronald Ching-wan Ma is a member of the international editorial board of
Figures



References
-
- Wang H, Li N, Chivese T, Werfalli M, Sun H, Yuen L, et al. IDF Diabetes Atlas: estimation of global and regional gestational diabetes mellitus prevalence for 2021 by International Association of Diabetes in Pregnancy Study Group’s criteria. Diabetes Res Clin Pract. 2022;183:109050. - PubMed
-
- HAPO Study Cooperative Research Group. Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
- R01 HD034242/HD/NICHD NIH HHS/United States
- T32 CA009302/CA/NCI NIH HHS/United States
- T12-402/13 N/RGC Theme-based Research Scheme
- 471713/Council of the Hong Kong SAR
- R01-HD34243/DK/NIDDK NIH HHS/United States
- 14102719/Council of the Hong Kong SAR
- University Grants Committee Research Grants Matching Scheme
- R4012-18/Research Impact Fund
- 14118718/Council of the Hong Kong SAR
- R01-HD34242/National Institute of Child Health and Human Development
- 473408/Council of the Hong Kong SAR
- R01 HD034243/HD/NICHD NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

