foxg1a is required for hair cell development and regeneration in the zebrafish lateral line
- PMID: 39301848
- PMCID: PMC11423914
- DOI: 10.1242/bio.060580
foxg1a is required for hair cell development and regeneration in the zebrafish lateral line
Abstract
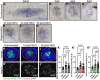
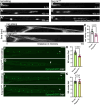
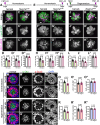
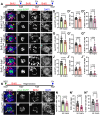
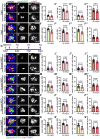
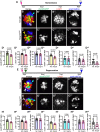
Mechanosensory hair cells located in the inner ear mediate the sensations of hearing and balance. If damaged, mammalian inner ear hair cells are unable to regenerate, resulting in permanent sensory deficits. Aquatic vertebrates like zebrafish (Danio rerio) have a specialized class of mechanosensory hair cells found in the lateral line system, allowing them to sense changes in water current. Unlike mammalian inner ear hair cells, lateral line hair cells can robustly regenerate following damage. In mammals, the transcription factor Foxg1 functions to promote normal development of the inner ear. Foxg1a is expressed in lateral line sensory organs in zebrafish larvae, but its function during lateral line development and regeneration has not been investigated. Our study demonstrates that mutation of foxg1a results in slower posterior lateral line primordium migration and delayed neuromast formation. In developing and regenerating neuromasts, we find that loss of Foxg1a function results in reduced hair cell numbers, as well as decreased proliferation of neuromast cells. Foxg1a specifically regulates the development and regeneration of Islet1-labeled hair cells. These data suggest that Foxg1 may be a valuable target for investigation of clinical hair cell regeneration.
Keywords: foxg1a; Development; Hair cells; Proliferation; Zebrafish.
© 2024. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures


References
-
- Baek, S., Tran, N. T. T., Diaz, D. C., Tsai, Y. Y., Acedo, J. N., Lush, M. E. and Piotrowski, T. (2022). Single-cell transcriptome analysis reveals three sequential phases of gene expression during zebrafish sensory hair cell regeneration. Dev. Cell 57, 799-819.e796. 10.1016/j.devcel.2022.03.001 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

