Morphogens in the evolution of size, shape and patterning
- PMID: 39302048
- PMCID: PMC7616732
- DOI: 10.1242/dev.202412
Morphogens in the evolution of size, shape and patterning
Abstract
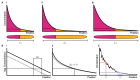
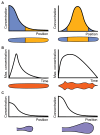
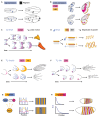
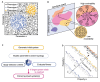
Much of the striking diversity of life on Earth has arisen from variations in the way that the same molecules and networks operate during development to shape and pattern tissues and organs into different morphologies. However, we still understand very little about the potential for diversification exhibited by different, highly conserved mechanisms during evolution, or, conversely, the constraints that they place on evolution. With the aim of steering the field in new directions, we focus on morphogen-mediated patterning and growth as a case study to demonstrate how conserved developmental mechanisms can adapt during evolution to drive morphological diversification and optimise functionality, and to illustrate how evolution algorithms and computational tools can be used alongside experiments to provide insights into how these conserved mechanisms can evolve. We first introduce key conserved properties of morphogen-driven patterning mechanisms, before summarising comparative studies that exemplify how changes in the spatiotemporal expression and signalling levels of morphogens impact the diversification of organ size, shape and patterning in nature. Finally, we detail how theoretical frameworks can be used in conjunction with experiments to probe the role of morphogen-driven patterning mechanisms in evolution. We conclude that morphogen-mediated patterning is an excellent model system and offers a generally applicable framework to investigate the evolution of developmental mechanisms.
Keywords: Evolution; GRNs; Morphogens; Patterning.
© 2024. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures
References
-
- Abzhanov A, Protas M, Grant BR, Grant PR, Tabin CJ. BMP4 and morphological variation of beaks in Darwin’s finches. Science. 2004;305(5689):1462–1465. - PubMed
-
- Adler M, Szekely P, Mayo A, Alon U. Optimal regulatory circuit topologies for fold-change detection. Cell Syst. 2017;4(2):171–181. - PubMed
-
- Aguilar-Hidalgo D, Werner S, Wartlick O, González-Gaitán M, Friedrich BM, Jülicher F. Critical point in self-organised tissue growth. Phys Rev Lett. 2018;120(19):198102. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

