Lipoprotein(a) and cardiovascular disease
- PMID: 39302109
- PMCID: PMC11555715
- DOI: 10.1042/BCJ20240037
Lipoprotein(a) and cardiovascular disease
Abstract
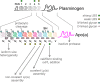
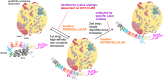
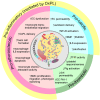
Elevated plasma levels of lipoprotein(a) (Lp(a)) are a prevalent, independent, and causal risk factor for atherosclerotic cardiovascular disease and calcific aortic valve disease. Lp(a) consists of a lipoprotein particle resembling low density lipoprotein and the covalently-attached glycoprotein apolipoprotein(a) (apo(a)). Novel therapeutics that specifically and potently lower Lp(a) levels are currently in advanced stages of clinical development, including in large, phase 3 cardiovascular outcomes trials. However, fundamental unanswered questions remain concerning some key aspects of Lp(a) biosynthesis and catabolism as well as the true pathogenic mechanisms of the particle. In this review, we describe the salient biochemical features of Lp(a) and apo(a) and how they underlie the disease-causing potential of Lp(a), the factors that determine plasma Lp(a) concentrations, and the mechanism of action of Lp(a)-lowering drugs.
Keywords: apolipoprotein(a); atherosclerotic cardiovascular disease; calcific aortic valve disease; lipoprotein metabolism; lipoprotein(a); thrombosis.
© 2024 The Author(s).
Conflict of interest statement
M.B. Boffa has no competing interests to declare. M.L. Koschinsky holds a research contract with Eli Lilly & Co. and is a consultant for Novartis and Eli Lilly & Co.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

