Single housing of juveniles accelerates early-stage growth but extends adult lifespan in African turquoise killifish
- PMID: 39302208
- PMCID: PMC11466477
- DOI: 10.18632/aging.206111
Single housing of juveniles accelerates early-stage growth but extends adult lifespan in African turquoise killifish
Abstract
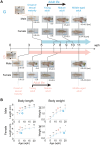
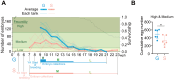
Within the same species, individuals exhibiting faster growth tend to have shorter lifespans, even if their fast growth arises from early-life pharmacological interventions. However, in vertebrates, the impact of the early-life environment on the growth rate and lifespan has not been fully elucidated. In this study, by utilizing the short-lived African turquoise killifish, which is suitable for a comprehensive life-stage analysis in a brief timeframe, we explored the effects of housing density during the juvenile stage on holistic life traits. As a result, we found that lower housing densities resulted in faster growth, but led to longer adult lifespan, which was contrary to the common notion. Furthermore, the single-housed adult fish displayed a longer egg-laying period than did their group-housed counterparts. Our transcriptome analysis also demonstrated that, in terms of internal transcriptional programs, the life stage progression and aging process of single-housed fish were slower than those of group-housed fish. Collectively, our results suggest that sharing housing with others in early life might influence whole-life attributes, potentially leading to specific life history traits beyond the typical relationship between the growth rate and lifespan.
Keywords: African turquoise killifish; aging; growth; housing density; lifespan.
Conflict of interest statement
Figures





References
MeSH terms
Supplementary concepts
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

