Disease extent according to baseline [18F]fluorodeoxyglucose PET/CT and molecular subtype: prediction of axillary treatment response after neoadjuvant systemic therapy for breast cancer
- PMID: 39302345
- PMCID: PMC11414043
- DOI: 10.1093/bjs/znae203
Disease extent according to baseline [18F]fluorodeoxyglucose PET/CT and molecular subtype: prediction of axillary treatment response after neoadjuvant systemic therapy for breast cancer
Abstract
Background: Axillary disease extent according to baseline [18F]fluorodeoxyglucose PET/CT combined with pathological axillary treatment response has been proposed to guide de-escalation of axillary treatment for clinically node-positive breast cancer patients treated with neoadjuvant systemic therapy. The aim of this study was to assess whether axillary disease extent according to baseline [18F]fluorodeoxyglucose PET/CT and breast cancer molecular subtype are predictors of axillary pCR.
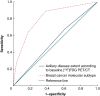
Methods: This study included clinically node-positive patients treated with neoadjuvant systemic therapy in the prospective Radioactive Iodine Seed placement in the Axilla with Sentinel lymph node biopsy ('RISAS') trial (NCT02800317) with baseline [18F]fluorodeoxyglucose PET/CT imaging available. The predictive value of axillary disease extent according to baseline [18F]fluorodeoxyglucose PET/CT and breast cancer molecular subtype to estimate axillary pCR was evaluated using logistic regression analysis. Discriminative ability is expressed using ORs with 95% confidence intervals.
Results: Overall, 185 patients were included, with an axillary pCR rate of 29.7%. The axillary pCR rate for patients with limited versus advanced baseline axillary disease according to [18F]fluorodeoxyglucose PET/CT was 31.9% versus 26.1% respectively. Axillary disease extent was not a significant predictor of axillary pCR (OR 0.75 (95% c.i. 0.38 to 1.46) (P = 0.404)). There were significant differences in axillary pCR rates between breast cancer molecular subtypes. The lowest probability (7%) was found for hormone receptor+/human epidermal growth factor receptor 2- tumours. Using this category as a reference group, significantly increased ORs of 14.82 for hormone receptor+/human epidermal growth factor receptor 2+ tumours, 40 for hormone receptor-/human epidermal growth factor receptor 2+ tumours, and 6.91 for triple-negative tumours were found (P < 0.001).
Conclusion: Molecular subtype is a significant predictor of axillary pCR after neoadjuvant systemic therapy, whereas axillary disease extent according to baseline [18F]fluorodeoxyglucose PET/CT is not.
© The Author(s) 2024. Published by Oxford University Press on behalf of BJS Foundation Ltd.
Figures
References
-
- Cao S, Liu X, Cui J, Liu X, Zhong J, Yang Z et al. Feasibility and reliability of sentinel lymph node biopsy after neoadjuvant chemotherapy in breast cancer patients with positive axillary nodes at initial diagnosis: an up-to-date meta-analysis of 3,578 patients. Breast 2021;59:256–269 - PMC - PubMed
-
- van Nijnatten TJ, Simons JM, Smidt ML, van der Pol CC, van Diest PJ, Jager A et al. A novel less-invasive approach for axillary staging after neoadjuvant chemotherapy in patients with axillary node-positive breast cancer by combining radioactive iodine seed localization in the axilla with the sentinel node procedure (RISAS): a Dutch prospective multicenter validation study. Clin Breast Cancer 2017;17:399–402 - PubMed
-
- Esgueva A, Siso C, Espinosa-Bravo M, Sobrido C, Miranda I, Salazar JP et al. Leveraging the increased rates of pathologic complete response after neoadjuvant treatment in breast cancer to de-escalate surgical treatments. J Surg Oncol 2021;123:71–79 - PubMed
-
- Hong J, Tong Y, He J, Chen X, Shen K. Association between tumour molecular subtype, clinical stage and axillary pathological response in breast cancer patients undergoing complete pathological remission after neoadjuvant chemotherapy: potential implications for de-escalation of axillary surgery. Ther Adv Med Oncol 2021;13:1758835921996673. - PMC - PubMed
-
- Samiei S, van Nijnatten TJ, de Munck L, Keymeulen KB, Simons JM, Kooreman LF et al. Correlation between pathologic complete response in the breast and absence of axillary lymph node metastases after neoadjuvant systemic therapy. Ann Surg 2020;271:574–580 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

