Advancements and Challenges in Organic-Inorganic Composite Solid Electrolytes for All-Solid-State Lithium Batteries
- PMID: 39302512
- PMCID: PMC11415606
- DOI: 10.1007/s40820-024-01498-y
Advancements and Challenges in Organic-Inorganic Composite Solid Electrolytes for All-Solid-State Lithium Batteries
Abstract
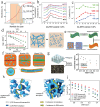
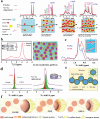
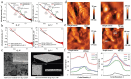
To address the limitations of contemporary lithium-ion batteries, particularly their low energy density and safety concerns, all-solid-state lithium batteries equipped with solid-state electrolytes have been identified as an up-and-coming alternative. Among the various SEs, organic-inorganic composite solid electrolytes (OICSEs) that combine the advantages of both polymer and inorganic materials demonstrate promising potential for large-scale applications. However, OICSEs still face many challenges in practical applications, such as low ionic conductivity and poor interfacial stability, which severely limit their applications. This review provides a comprehensive overview of recent research advancements in OICSEs. Specifically, the influence of inorganic fillers on the main functional parameters of OICSEs, including ionic conductivity, Li+ transfer number, mechanical strength, electrochemical stability, electronic conductivity, and thermal stability are systematically discussed. The lithium-ion conduction mechanism of OICSE is thoroughly analyzed and concluded from the microscopic perspective. Besides, the classic inorganic filler types, including both inert and active fillers, are categorized with special emphasis on the relationship between inorganic filler structure design and the electrochemical performance of OICSEs. Finally, the advanced characterization techniques relevant to OICSEs are summarized, and the challenges and perspectives on the future development of OICSEs are also highlighted for constructing superior ASSLBs.
Keywords: Characterization techniques; Composite solid electrolytes; Inorganic filler; Interfacial stability; Li-ion conduction mechanism.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no interest conflict. They have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures











Similar articles
-
Engineering Physicochemical Properties of Nanofillers for High-Performance Composite Solid-State Electrolytes in Lithium Metal Batteries.ACS Appl Mater Interfaces. 2025 Jun 25;17(25):36763-36772. doi: 10.1021/acsami.5c05964. Epub 2025 Jun 12. ACS Appl Mater Interfaces. 2025. PMID: 40501202
-
Recent advances in garnet-based electrolytes for solid-state lithium metal batteries: interfacial challenges and engineering strategies.Mater Horiz. 2025 Aug 11;12(16):6082-6123. doi: 10.1039/d5mh00583c. Mater Horiz. 2025. PMID: 40397013 Review.
-
An ultrathin polymer-based solid-state electrolyte membrane with high conductivity for lithium metal batteries operable at -25 °C.J Colloid Interface Sci. 2025 Nov 15;698:138071. doi: 10.1016/j.jcis.2025.138071. Epub 2025 Jun 2. J Colloid Interface Sci. 2025. PMID: 40472460
-
Ceramic-Polymer Composite Solid-State Electrolytes for Solid-State Lithium Metal Batteries: Mechanism, Strategy, and Prospect.Small. 2025 Jun;21(24):e2503743. doi: 10.1002/smll.202503743. Epub 2025 May 2. Small. 2025. PMID: 40317830 Review.
-
Designing High-Ionic-Conductivity and Air-Stable Composite Sulfide Electrolytes via Polymer-in-Salt Binder with Surface Engineering for All-Solid-State Lithium Batteries.Small. 2025 Jun 26:e2503875. doi: 10.1002/smll.202503875. Online ahead of print. Small. 2025. PMID: 40567046
Cited by
-
In Situ Polymerization in COF Boosts Li-Ion Conduction in Solid Polymer Electrolytes for Li Metal Batteries.Nanomicro Lett. 2025 May 6;17(1):248. doi: 10.1007/s40820-025-01768-3. Nanomicro Lett. 2025. PMID: 40327199 Free PMC article.
-
Composite Polymer Electrolyte Based on PAN/TPU for Lithium-Ion Batteries Operating at Room Temperature.Polymers (Basel). 2024 Nov 25;16(23):3280. doi: 10.3390/polym16233280. Polymers (Basel). 2024. PMID: 39684025 Free PMC article.
-
Lithium-Ion Dynamic Interface Engineering of Nano-Charged Composite Polymer Electrolytes for Solid-State Lithium-Metal Batteries.Nanomicro Lett. 2025 Aug 29;18(1):46. doi: 10.1007/s40820-025-01899-7. Nanomicro Lett. 2025. PMID: 40879855 Free PMC article.
-
Host-Guest Inversion Engineering Induced Superionic Composite Solid Electrolytes for High-Rate Solid-State Alkali Metal Batteries.Nanomicro Lett. 2025 Mar 17;17(1):190. doi: 10.1007/s40820-025-01691-7. Nanomicro Lett. 2025. PMID: 40091117 Free PMC article.
-
Artificial Intelligence Empowers Solid-State Batteries for Material Screening and Performance Evaluation.Nanomicro Lett. 2025 Jun 6;17(1):287. doi: 10.1007/s40820-025-01797-y. Nanomicro Lett. 2025. PMID: 40478305 Free PMC article. Review.
References
-
- M. Li, J. Lu, Z. Chen, K. Amine, 30 years of lithium-ion batteries. Adv. Mater. 30, 1800561 (2018). 10.1002/adma.201800561 - PubMed
-
- J.M. Tarascon, M. Armand, Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2001). 10.1038/35104644 - PubMed
-
- L. Wang, T. Liu, T. Wu, J. Lu, Strain-retardant coherent perovskite phase stabilized Ni-rich cathode. Nature 611, 61–67 (2022). 10.1038/s41586-022-05238-3 - PubMed
-
- Y. Zhang, W. Zhao, C. Kang, S. Geng, J. Zhu et al., Phase-junction engineering triggered built-in electric field for fast-charging batteries operated at −30 °C. Matter 6, 1928–1944 (2023). 10.1016/j.matt.2023.03.026
Publication types
LinkOut - more resources
Full Text Sources
