Wearable Device-Based Intervention for Promoting Patient Physical Activity After Lung Cancer Surgery: A Nonrandomized Clinical Trial
- PMID: 39302678
- PMCID: PMC11415788
- DOI: 10.1001/jamanetworkopen.2024.34180
Wearable Device-Based Intervention for Promoting Patient Physical Activity After Lung Cancer Surgery: A Nonrandomized Clinical Trial
Abstract
Importance: Emerging evidence suggests that wearable devices are feasible for monitoring physical activity among patients with lung cancer. However, the association between wearable devices and improvement in patient recovery after surgery remains underexplored.
Objective: To evaluate the effects of a wearable device intervention on the recovery of physical activity, cardiopulmonary function, and health-related quality of life (HRQOL) after lung cancer surgery.
Design, setting, and participants: This nonrandomized clinical trial with a historical control was conducted at a single tertiary cancer center (Samsung Comprehensive Cancer Center) in Seoul, South Korea, between October 18, 2018, and May 24, 2019. Patients were included if they had suspected or confirmed non-small cell lung cancer scheduled for curative surgery more extensive than lobectomy and had an Eastern Cooperative Oncology Group status of 0 or 1. Patients were compared with historical control participants from data collected between September 20, 2017, and September 10, 2018, as part of the Coordinated Approach to Cancer Patients' Health for Lung Cancer (CATCH-LUNG) prospective cohort study. Data analysis was performed between June 21 and July 16, 2020.
Intervention: A personalized exercise regimen monitored via a wearable device was administered to intervention patients at home in 3 stages: preoperative (from diagnosis to surgery), immediate (from discharge to 2 months after surgery), and later postoperative (from 2 to 6 months after surgery). Control patients received usual care.
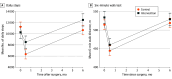
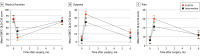
Main outcomes and measures: The primary outcome was cardiopulmonary function, and the co-primary outcome was physical activity at 6 months after surgery, measured with 6-minute walking distance (6MWD) and number of daily steps, using a linear regression model. Secondary outcomes were changes in cardiopulmonary function, physical activity, and HRQOL, including function and symptoms from baseline to 2 weeks and 6 months after surgery. Additionally, cardiopulmonary function and physical activity (number of daily steps and time spent on moderate-to-vigorous physical activity [MVPA]) at 2 weeks after surgery, physical activity (time spent on MVPA) at 6 months after surgery, and HRQOL, including function and symptoms at 2 weeks and 6 months after surgery, were assessed as secondary outcomes.
Results: This trial included 74 patients in the intervention group (mean [SD] age, 60.4 [8.7] years; 31 [41.9%] men and 43 [58.1%] women) and 120 in the control group (mean [SD] age, 60.2 [8.7] years; 65 [54.2%] men and 55 [45.8%] women). Daily steps, MVPA, and 6MWD decreased initially at 2 weeks after surgery but increased thereafter. The control group had a larger decrease in the number of daily steps from baseline compared with the intervention group (-4877 [95% CI, -5861 to -3893] steps vs -1753 [95% CI, -2968 to -539] steps) at 2 weeks after surgery. By 6 months after surgery, the intervention group increased their daily steps by 2220 (95% CI, 1006 to 3435) from baseline, whereas the control group did not return to their baseline number of steps. The intervention group had significantly more daily steps (12 321 [95% CI, 8749-15 761] vs 10 118 [95% CI, 7341-13 420]; P = .007) and had greater vigorous physical activity (33.6 [95% CI, 13.5 to 59.8] vs 18.5 [5.7 to 40.8] minutes; P = .003) at 6 months after surgery compared with the control group. No difference in 6MWD was found. However, the intervention group had better patient-reported physical function (mean [SD] score, 82.2 [17.3] vs 76.9 [17.5]; P = .04), less dyspnea (mean [SD] score, 24.8 [27.1] vs 34.5 [31.6]; P = .03), and less pain (mean [SD] score, 21.4 [20.2] vs 30.1 [26.8]; P = .01) at 2 weeks after surgery and less dyspnea (mean [SD] score, 5.4[12.4] vs 12[23.3]; P = .01) at 6 months after surgery compared with the control group.
Conclusions and relevance: In this nonrandomized clinical trial, integration of perioperative exercise interventions using wearable devices improved physical activity (especially MVPA) and dyspnea at 6 months after lung cancer surgery compared with usual care. This finding suggests a promising role for wearable devices in personalizing perioperative rehabilitation strategies.
Trial registration: ClinicalTrials.gov Identifier: NCT03215537.
Conflict of interest statement
Figures
Comment in
-
Implementing Wearable Devices in Postoperative Care.JAMA Netw Open. 2024 Sep 3;7(9):e2434124. doi: 10.1001/jamanetworkopen.2024.34124. JAMA Netw Open. 2024. PMID: 39302682 No abstract available.
References
-
- Goldstraw P, Chansky K, Crowley J, et al. ; International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions; International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee Advisory Boards and Participating Institutions . The IASLC Lung Cancer Staging Project: proposals for revision of the TNM stage groupings in the forthcoming (eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39-51. doi: 10.1016/j.jtho.2015.09.009 - DOI - PubMed

