Global freshwater distribution of Telonemia protists
- PMID: 39303138
- PMCID: PMC11512789
- DOI: 10.1093/ismejo/wrae177
Global freshwater distribution of Telonemia protists
Abstract
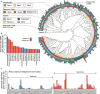
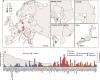
Telonemia are one of the oldest identified marine protists that for most part of their history have been recognized as a distinct incertae sedis lineage. Today, their evolutionary proximity to the SAR supergroup (Stramenopiles, Alveolates, and Rhizaria) is firmly established. However, their ecological distribution and importance as a natural predatory flagellate, especially in freshwater food webs, still remain unclear. To unravel the distribution and diversity of the phylum Telonemia in freshwater habitats, we examined over a thousand freshwater metagenomes from all over the world. In addition, to directly quantify absolute abundances, we analyzed 407 samples from 97 lakes and reservoirs using Catalyzed Reporter Deposition-Fluorescence in situ Hybridization (CARD-FISH). We recovered Telonemia 18S rRNA gene sequences from hundreds of metagenomic samples from a wide variety of habitats, indicating a global distribution of this phylum. However, even after this extensive sampling, our phylogenetic analysis did not reveal any new major clades, suggesting current molecular surveys are near to capturing the full diversity within this group. We observed excellent concordance between CARD-FISH analyses and estimates of abundances from metagenomes. Both approaches suggest that Telonemia are largely absent from shallow lakes and prefer to inhabit the colder hypolimnion of lakes and reservoirs in the Northern Hemisphere, where they frequently bloom, reaching 10%-20% of the total heterotrophic flagellate population, making them important predatory flagellates in the freshwater food web.
Keywords: CARD-FISH; Telonemia; freshwater lakes; metagenomics; microbial food webs; predatory flagellate.
© The Author(s) 2024. Published by Oxford University Press on behalf of the International Society for Microbial Ecology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Sommer U, Adrian R, De Senerpont DL et al. Beyond the plankton ecology group (PEG) model: mechanisms driving plankton succession. Annu Rev Ecol Evol Syst 2012;43:429–48. 10.1146/annurev-ecolsys-110411-160251 - DOI
-
- Šimek K, Nedoma J, Znachor P et al. A finely tuned symphony of factors modulates the microbial food web of a freshwater reservoir in spring. Limnol Oceanogr 2014;59:1477–92. 10.4319/lo.2014.59.5.1477 - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

