Canagliflozin and iron metabolism in the CREDENCE trial
- PMID: 39304530
- PMCID: PMC11960735
- DOI: 10.1093/ndt/gfae198
Canagliflozin and iron metabolism in the CREDENCE trial
Abstract
Background: Studies in patients with heart failure have indicated that sodium-glucose cotransporter 2 (SGLT2) inhibitors increase iron use and enhance erythropoiesis. In this post hoc analysis of the Canagliflozin and Renal Endpoints in Diabetes with Established Nephropathy Clinical Evaluation (CREDENCE) trial, we evaluated the effects of canagliflozin on iron metabolism in patients with chronic kidney disease (CKD) and whether the effects of canagliflozin on hemoglobin and cardiorenal outcomes were modified by iron deficiency.
Methods: We measured serum iron, total iron binding capacity (TIBC), transferrin saturation (TSAT) and ferritin at baseline and 12 months. The effects of canagliflozin, relative to placebo, on iron markers were assessed with analysis of covariance. Interactions between baseline iron deficiency, defined as TSAT <20%, and the effects of canagliflozin on hemoglobin and cardiorenal outcomes were evaluated with mixed effect models and Cox regression models, respectively.
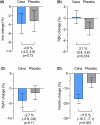
Results: Of 4401 participants randomized in CREDENCE, 2416 (54.9%) had iron markers measured at baseline, of whom 924 (38.2%) were iron deficient. Canagliflozin, compared with placebo, increased TIBC by 2.1% [95% confidence interval (CI) 0.4, 3.8; P = .014] and decreased ferritin by 11.5% (95% CI 7.1, 15.7; P < .001) with no clear effect on serum iron or TSAT. Canagliflozin increased hemoglobin over the trial duration by 7.3 g/L (95% CI 6.2, 8.5; P < .001) and 6.7 g/L (95% CI 5.2, 8.2; P < .001) in patients with and without iron deficiency, respectively (P for interaction = .38). The relative effect of canagliflozin on the primary outcome of doubling of serum creatinine, kidney failure or death due to cardiovascular disease or kidney failure (hazard ratio 0.70, 95% CI 0.56, 0.87) was consistent regardless of iron deficiency (P for interaction = .83), as were effects on other cardiovascular and mortality outcomes (all P for interactions ≥0.10).
Conclusion: Iron deficiency is highly prevalent in patients with type 2 diabetes and CKD. Canagliflozin increased TIBC and decreased ferritin in patients with type 2 diabetes and CKD, suggesting increased iron utilization, and improved hemoglobin levels and clinical outcomes regardless of iron deficiency.
Keywords: anemia; cardiovascular; chronic kidney disease; iron deficiency; sodium-glucose cotransporter 2 inhibitors.
© The Author(s) 2024. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
A.K., N.J. and T.W. have no conflicts of interest. H.J.L.H. is supported by a VIDI (917.15.306) grant from the Netherlands Organization for Scientific Research and has served as a consultant for AbbVie, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, CSL Pharma, Fresenius, Gilead, Janssen, Merck, Mundipharma, Mitsubishi Tanabe and Retrophin; and has received grant support from AbbVie, AstraZeneca, Boehringer Ingelheim and Janssen. C.A. is supported by an NHMRC/Medical Research Future Fund Priority Investigator Grant and an NSW Health Early to Mid Career Research Grant, and is an employee of the George Institute for Global Health. She is responsible for the secondary analysis program for the CANVAS Program and the CREDENCE trial. She has received honoraria, travel grants, and served on Advisory Boards for AstraZeneca, Novo Nordisk and Amgen. B.N. is supported by a National Health and Medical Research Council Investigator Grant (APP1197709). His institution has received grants, travel and consulting fees from Janssen pharmaceuticals for work done on CANVAS and CREDENCE. M.J. is responsible for research projects that have received funding from Amgen, Baxter, CSL, Dimerix, Eli Lilly, Gambro and MSD, and has received advisory, steering committee and/or speaker fees from Akebia, Amgen, AstraZeneca, Baxter, Bayer, Boehringer Ingelheim, Cesas Medical, Chinook, CSL, Janssen, Medcon International, Medscape, MSD, Occuryx, Roche and Vifor, with any consultancy, honoraria or travel support paid to her institution. S.V.B. has received speaking fees from Bayer, Pfizer and Vifor Pharma; has attended advisory boards for AstraZeneca, Bayer and Vifor Pharma; and has received non-financial research support from Bayer. All honoraria received were paid to S.V.B.’s institution. K.W.M. has received research support from Afferent, Amgen, Apple, Inc., AstraZeneca, Cardiva Medical, Inc., Daiichi, Ferring, Google (Verily), Johnson & Johnson, Luitpold, Medtronic, Merck, National Institutes of Health, Novartis, Sanofi, St Jude and Tenax. He also has served as a consultant (speaker fees for continuing medical education events only) for Abbott, Ablynx, AstraZeneca, Baim Institute, Boehringer Ingelheim, Bristol-Myers Squibb, Elsevier, GlaxoSmithKline, Johnson & Johnson, MedErgy, Medscape, Mitsubishi, Myokardia, National Institutes of Health, Novartis, Novo Nordisk, Portola, Radiometer, Regeneron, Springer Publishing and University of California, San Francisco. C.P. has served on the steering committee for CREDENCE, has been a speaker for Janssen Cilag, an advisory board member and speaker for AstraZeneca, and a speaker for Eli Lilly and Boehringer Ingelheim. V.P. reports receiving honoraria for scientific presentations and/or advisory board attendance from Abbvie, Amgen, AstraZeneca, Bayer, Baxter, Boehringer Ingelheim, Chinook, Durect, Eli Lilly, Gilead, GSK, Janssen, Merck, Mitsubishi Tanabe, Mundipharma, Novartis, Novo Nordisk, Pharmalink, Pfizer, Reata, Relypsa, Roche, Sanofi and Servier. M.K.H. is an employee of Janssen Research & Development, LLC. S.J.L.B. reports research funding from Astellas Pharma and Chiesi Pharmaceuticals BV and an advisory or leadership role with the Dutch Health Council and the scientific board of the Dutch Kidney Foundation. B.L.N. has received fees for advisory boards, scientific presentations, speaker fees, steering committee roles and travel support from the American Diabetes Association, Alexion, AstraZeneca, Bayer, Boehringer and Ingelheim, Cambridge Healthcare Research, Cornerstone Medical Education, the Limbic, Janssen and Medscape, with all honoraria paid to his institution.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

