Interlimb coordination is not strictly controlled during walking
- PMID: 39304734
- PMCID: PMC11415362
- DOI: 10.1038/s42003-024-06843-w
Interlimb coordination is not strictly controlled during walking
Abstract
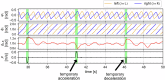
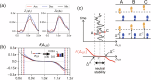
In human walking, the left and right legs move alternately, half a stride out of phase with each other. Although various parameters, such as stride frequency and length, vary with walking speed, the antiphase relationship remains unchanged. In contrast, during walking in left-right asymmetric situations, the relative phase shifts from the antiphase condition to compensate for the asymmetry. Interlimb coordination is important for adaptive walking and we expect that interlimb coordination is strictly controlled during walking. However, the control mechanism remains unclear. In the present study, we derived a quantity that models the control of interlimb coordination during walking using two coupled oscillators based on the phase reduction theory and Bayesian inference method. The results were not what we expected. Specifically, we found that the relative phase is not actively controlled until the deviation from the antiphase condition exceeds a certain threshold. In other words, the control of interlimb coordination has a dead zone like that in the case of the steering wheel of an automobile. It is conjectured that such forgoing of control enhances energy efficiency and maneuverability. Our discovery of the dead zone in the control of interlimb coordination provides useful insight for understanding gait control in humans.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Alexander, R.McN. Principles of animal locomotion. Princeton University Press: Princeton, NJ (2003).
Publication types
MeSH terms
Grants and funding
- JPMJCR09U2/MEXT | JST | Core Research for Evolutional Science and Technology (CREST)
- JPMJCR09U2/MEXT | JST | Core Research for Evolutional Science and Technology (CREST)
- JP20K21810/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP20H04144/MEXT | Japan Society for the Promotion of Science (JSPS)
- JP20K20520/MEXT | Japan Society for the Promotion of Science (JSPS)

