Lysosomes in the immunometabolic reprogramming of immune cells in atherosclerosis
- PMID: 39304748
- PMCID: PMC11835540
- DOI: 10.1038/s41569-024-01072-4
Lysosomes in the immunometabolic reprogramming of immune cells in atherosclerosis
Abstract
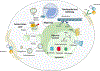
Lysosomes have a central role in the disposal of extracellular and intracellular cargo and also function as metabolic sensors and signalling platforms in the immunometabolic reprogramming of macrophages and other immune cells in atherosclerosis. Lysosomes can rapidly sense the presence of nutrients within immune cells, thereby switching from catabolism of extracellular material to the recycling of intracellular cargo. Such a fine-tuned degradative response supports the generation of metabolic building blocks through effectors such as mTORC1 or TFEB. By coupling nutrients to downstream signalling and metabolism, lysosomes serve as a crucial hub for cellular function in innate and adaptive immune cells. Lysosomal dysfunction is now recognized to be a hallmark of atherogenesis. Perturbations in nutrient-sensing and signalling have profound effects on the capacity of immune cells to handle cholesterol, perform phagocytosis and efferocytosis, and limit the activation of the inflammasome and other inflammatory pathways. Strategies to improve lysosomal function hold promise as novel modulators of the immunoinflammatory response associated with atherosclerosis. In this Review, we describe the crosstalk between lysosomal biology and immune cell function and polarization, with a particular focus on cellular immunometabolic reprogramming in the context of atherosclerosis.
© 2024. Springer Nature Limited.
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
-
Liu GY & Sabatini DM mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 21, 183–203 (2020).,
In this review, the authors discuss the latest literature describing the intimate association between lysosomes and mTORC complexes that are required to regulate cellular metabolic fate in states of nutrition or starvation in health and disease.
-
-
- Platt FM, d’Azzo A, Davidson BL, Neufeld EF & Tifft CJ Lysosomal storage diseases. Nat. Rev. Dis. Prim. 4, 27 (2018). - PubMed
-
-
de Duve C. The participation of lysosomes in the transformation of smooth muscle cells to foamy cells in the aorta of cholesterol-fed rabbits. Acta Cardiol. Suppl 20, 9–25 (1974).,
Recognition of atherosclerotic plaque as a form of lysosomal storage diseases as a consequence of neutral lipid deposition in lysosomes of foam cells.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

