Neural precursor cells rescue symptoms of Rett syndrome by activation of the Interferon γ pathway
- PMID: 39304759
- PMCID: PMC11628625
- DOI: 10.1038/s44321-024-00144-9
Neural precursor cells rescue symptoms of Rett syndrome by activation of the Interferon γ pathway
Abstract
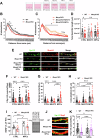
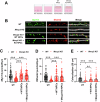
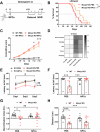
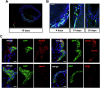
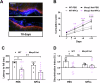
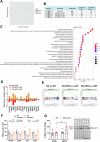
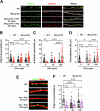
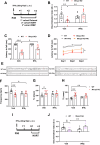
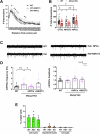
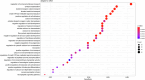
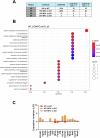
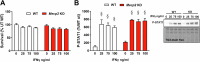
The beneficial effects of Neural Precursor Cell (NPC) transplantation in several neurological disorders are well established and they are generally mediated by the secretion of immunomodulatory and neurotrophic molecules. We therefore investigated whether Rett syndrome (RTT), that represents the first cause of severe intellectual disability in girls, might benefit from NPC-based therapy. Using in vitro co-cultures, we demonstrate that, by sensing the pathological context, NPC-secreted factors induce the recovery of morphological and synaptic defects typical of Mecp2 deficient neurons. In vivo, we prove that intracerebral transplantation of NPCs in RTT mice significantly ameliorates neurological functions. To uncover the molecular mechanisms underpinning the mediated benefic effects, we analyzed the transcriptional profile of the cerebellum of transplanted animals, disclosing the possible involvement of the Interferon γ (IFNγ) pathway. Accordingly, we report the capacity of IFNγ to rescue synaptic defects, as well as motor and cognitive alterations in Mecp2 deficient models, thereby suggesting this molecular pathway as a potential therapeutic target for RTT.
Keywords: Cytokine; Mecp2; Neurodevelopmental Disease; Stem Cells; Synapses.
© 2024. The Author(s).
Conflict of interest statement
Disclosure and competing interests statement. The authors declare no competing interests.
Figures


References
-
- Armstrong DD (2005) Neuropathology of Rett syndrome. J Child Neurol 20(9):747–753 - PubMed
-
- Arolt V, Rothermundt M, Wandinger KP, Kirchner H (2000) Decreased in vitro production of interferon-gamma and interleukin-2 in whole blood of patients with schizophrenia during treatment. Mol Psychiatry 5:150–158 - PubMed

