Super-silencer perturbation by EZH2 and REST inhibition leads to large loss of chromatin interactions and reduction in cancer growth
- PMID: 39304765
- PMCID: PMC11746141
- DOI: 10.1038/s41594-024-01391-7
Super-silencer perturbation by EZH2 and REST inhibition leads to large loss of chromatin interactions and reduction in cancer growth
Abstract
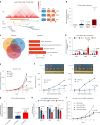
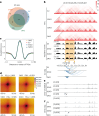
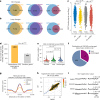
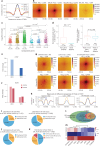
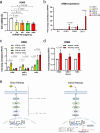
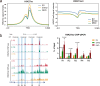
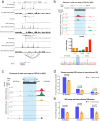
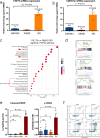
Human silencers have been shown to regulate developmental gene expression. However, the functional importance of human silencers needs to be elucidated, such as whether they can form 'super-silencers' and whether they are linked to cancer progression. Here, we show two silencer components of the FGF18 gene can cooperate through compensatory chromatin interactions to form a super-silencer. Double knockout of two silencers exhibited synergistic upregulation of FGF18 expression and changes in cell identity. To perturb the super-silencers, we applied combinational treatment of an enhancer of zeste homolog 2 inhibitor GSK343, and a repressor element 1-silencing transcription factor inhibitor, X5050 ('GR'). Interestingly, GR led to severe loss of topologically associated domains and loops, which were associated with reduced CTCF and TOP2A mRNA levels. Moreover, GR synergistically upregulated super-silencer-controlled genes related to cell cycle, apoptosis and DNA damage, leading to anticancer effects in vivo. Overall, our data demonstrated a super-silencer example and showed that GR can disrupt super-silencers, potentially leading to cancer ablation.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: M.J.F. declares seven patents held related to ChIA-PET and nucleic acid molecular biology methods. The other authors declare no competing interests.
Figures







References
-
- Pott, S. & Lieb, J. D. What are super-enhancers? Nat. Genet.47, 8–12 (2015). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

