A modular chemigenetic calcium indicator for multiplexed in vivo functional imaging
- PMID: 39304767
- PMCID: PMC11466818
- DOI: 10.1038/s41592-024-02411-6
A modular chemigenetic calcium indicator for multiplexed in vivo functional imaging
Abstract
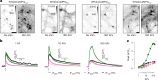
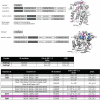
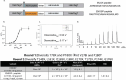
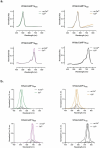
Genetically encoded fluorescent calcium indicators allow cellular-resolution recording of physiology. However, bright, genetically targetable indicators that can be multiplexed with existing tools in vivo are needed for simultaneous imaging of multiple signals. Here we describe WHaloCaMP, a modular chemigenetic calcium indicator built from bright dye-ligands and protein sensor domains. Fluorescence change in WHaloCaMP results from reversible quenching of the bound dye via a strategically placed tryptophan. WHaloCaMP is compatible with rhodamine dye-ligands that fluoresce from green to near-infrared, including several that efficiently label the brain in animals. When bound to a near-infrared dye-ligand, WHaloCaMP shows a 7× increase in fluorescence intensity and a 2.1-ns increase in fluorescence lifetime upon calcium binding. We use WHaloCaMP1a to image Ca2+ responses in vivo in flies and mice, to perform three-color multiplexed functional imaging of hundreds of neurons and astrocytes in zebrafish larvae and to quantify Ca2+ concentration using fluorescence lifetime imaging microscopy (FLIM).
© 2024. The Author(s).
Conflict of interest statement
H.F. and E.R.S. have filed patent applications on tryptophan-containing chemigenetic fluorescent indicators. L.D.L. and J.B.G. have filed patents and patent applications on fluorinated and azetidine-containing rhodamines. The other authors declare no competing interests.
Figures









Update of
-
A modular chemigenetic calcium indicator enables in vivo functional imaging with near-infrared light.bioRxiv [Preprint]. 2023 Jul 19:2023.07.18.549527. doi: 10.1101/2023.07.18.549527. bioRxiv. 2023. Update in: Nat Methods. 2024 Oct;21(10):1916-1925. doi: 10.1038/s41592-024-02411-6. PMID: 37503182 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

