TBK1-Zyxin signaling controls tumor-associated macrophage recruitment to mitigate antitumor immunity
- PMID: 39304793
- PMCID: PMC11535546
- DOI: 10.1038/s44318-024-00244-9
TBK1-Zyxin signaling controls tumor-associated macrophage recruitment to mitigate antitumor immunity
Abstract
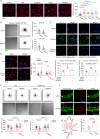
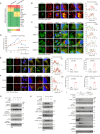
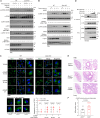
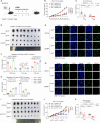
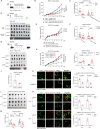
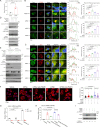
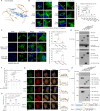
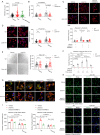
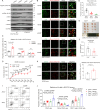
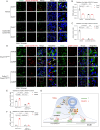
Mechanical control is fundamental for cellular localization within a tissue, including for tumor-associated macrophages (TAMs). While the innate immune sensing pathways cGAS-STING and RLR-MAVS impact the pathogenesis and therapeutics of malignant diseases, their effects on cell residency and motility remain incompletely understood. Here, we uncovered that TBK1 kinase, activated by cGAS-STING or RLR-MAVS signaling in macrophages, directly phosphorylates and mobilizes Zyxin, a key regulator of actin dynamics. Under pathological conditions and in STING or MAVS signalosomes, TBK1-mediated Zyxin phosphorylation at S143 facilitates rapid recruitment of phospho-Zyxin to focal adhesions, leading to subsequent F-actin reorganization and reduced macrophage migration. Intratumoral STING-TBK1-Zyxin signaling was evident in TAMs and critical in antitumor immunity. Furthermore, myeloid-specific or global disruption of this signaling decreased the population of CD11b+ F4/80+ TAMs and promoted PD-1-mediated antitumor immunotherapy. Thus, our findings identify a new biological function of innate immune sensing pathways by regulating macrophage tissue localization, thus providing insights into context-dependent mitigation of antitumor immunity.
Keywords: Antitumor Immunity; Cell Motility; TBK1; Tumor-associated Macrophages; cGAS-STING.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

