A randomized phase II trial on the addition of dutasteride to combined androgen blockade therapy versus combined androgen blockade therapy alone in patients with advanced or metastatic salivary duct carcinoma - the DUCT study protocol
- PMID: 39304797
- PMCID: PMC11415984
- DOI: 10.1186/s12885-024-12889-0
A randomized phase II trial on the addition of dutasteride to combined androgen blockade therapy versus combined androgen blockade therapy alone in patients with advanced or metastatic salivary duct carcinoma - the DUCT study protocol
Abstract
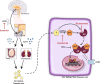
Background: Salivary duct carcinoma (SDC) is a rare and aggressive subtype of salivary gland cancer, frequently associated with incurable recurrences and distant metastases (R/M). Proliferation of SDC relies on androgen receptor (AR) signalling, prompting the use of combined androgen blockade (CAB, i.e., luteinizing hormone-releasing hormone agonist and/or AR antagonists) to R/M SDC patients. However, only a subset of patients benefits from such treatments. We have shown that response to CAB is associated with steroid 5α-reductase 1 (SRD5A1) mRNA expression. SRD5A1 catalyses the intracellular conversion of testosterone into the more potent AR-agonist dihydrotestosterone. This conversion can be inhibited by dutasteride, a potent SRD5A1-inhibitor, which is currently prescribed for benign prostatic hyperplasia. We hypothesize that repurposing dutasteride to target AR signalling in SDC could enhance therapeutic response and clinical outcome in SDC patients.
Methods: This prospective, open-label, randomized controlled phase II clinical trial, is designed to investigate whether dutasteride as an adjunct drug to CAB improves response rate and clinical outcome in patients with AR-positive R/M SDC. Patients are divided in two cohorts based on their prior systemic treatments. In cohort A, CAB-naïve patients (n = 74) will be randomly assigned to either a control arm (Arm 1) receiving CAB (goserelin 10.8 mg/3m and bicalutamide 50 mg/OD) or an experimental arm (Arm 2) where dutasteride (0.5 mg/OD) is added to the CAB regimen. In cohort B, patients with disease progression after adjuvant or first-line palliative CAB therapy (max. n = 24) will receive goserelin, bicalutamide, and dutasteride to assess whether the addition of dutasteride can overcome therapy resistance. The primary endpoints are the objective response rate and duration of response. Secondary endpoints are progression-free survival, overall survival, clinical benefit rate, quality of life, and safety. Translational research will be performed to explore molecular target expression differences and their correlation with clinical outcome.
Discussion: The DUCT study addresses an unmet medical need by investigating the repurposing of dutasteride to enhance treatment response and improve clinical outcome for patients with R/M SDC, especially those with limited alternative treatment options, such as HER2-negative cases. By repurposing a registered low-cost drug, this trial's findings could be readily applied into clinical practice.
Trial registration: Clinicaltrials.gov Identifier: NCT05513365. Date of registration: August 24, 2022.
Protocol version: Current protocol version 4.0, February 21, 2024.
Keywords: Androgen Deprivation Therapy; Androgen Receptor; Anti-hormonal therapy; Combined Androgen Blockade; Dutasteride; Rare Cancer; Salivary Duct Carcinoma; Salivary gland cancer; Steroid 5α-reductase; Systemic therapy.
© 2024. The Author(s).
Conflict of interest statement
JW, GV, GL, AvEvG, CD, MJ: No competing interests.
NvE: Research grants (institution): Astellas and Ipsen.
JS: Speakers honorarium: ‘Astellas, Bayer.’
CvH: Advisory (institution): Bayer, Bristol-Myers Squibb, Ipsen, MSD, Regeneron,. Research grant (institution): Astra Zeneca, Bristol-Myers Squibb, MSD, Merck, Ipsen, Novartis, and Sanofi.
Figures

References
-
- Boon E, Bel M, van Boxtel W, van der Graaf WTA, van Es RJJ, Eerenstein SEJ, Baatenburg de Jong RJ, van den Brekel MWM, van der Velden LA, Witjes MJH, et al. A clinicopathological study and prognostic factor analysis of 177 salivary duct carcinoma patients from The Netherlands. Int J Cancer. 2018;143(4):758–66. - DOI - PMC - PubMed
-
- WHO Classification of Tumours Editioral Board: Head and Neck Tumours, vol. 9, 5th edn. Lyon, France: International Agency for Research on Cancer; 2024.
-
- Fushimi C, Tada Y, Takahashi H, Nagao T, Ojiri H, Masubuchi T, Matsuki T, Miura K, Kawakita D, Hirai H, et al. A prospective phase II study of combined androgen blockade in patients with androgen receptor-positive metastatic or locally advanced unresectable salivary gland carcinoma. Ann Oncol. 2018;29(4):979–84. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

